Fig. 2. Single-component gas adsorption properties.
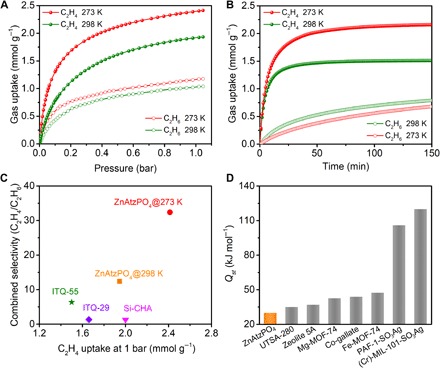
(A) Single-component adsorption isotherms of C2H4 and C2H6 on ZnAtzPO4 under 298 and 273 K. (B) Time-dependent gas uptake profiles of C2H4 and C2H6 at 0.4 bar and different temperatures. (C) Equilibrium-kinetic combined selectivity (C2H4/C2H6) and C2H4 uptake of ZnAtzPO4, ITQ-29, Si-CHA, and ITQ-55. Data for ITQ-55 were collected at 303 K, and those for ITQ-29 and Si-CHA were collected at 301 K. (D) Highest value of Qst calculated for C2H4 on ZnAtzPO4, UTSA-280, zeolite 5A, Mg-MOF-74, Co-gallate, Fe-MOF-74, PAF-1-SO3Ag, and (Cr)-MIL-101-SO3Ag.
