Figure 3. Effects of microglial activation on metabolism.
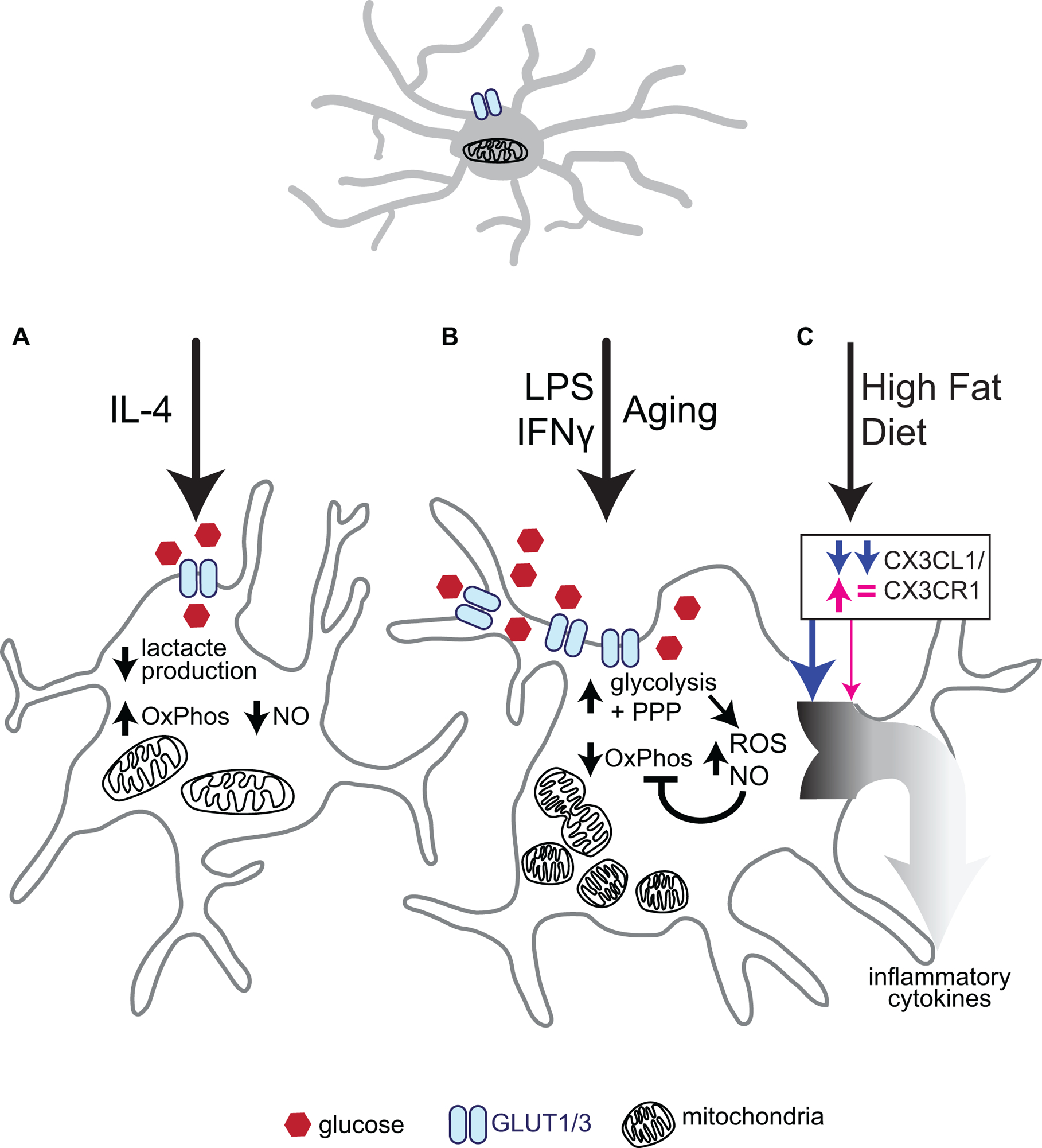
(A) Stimulation with the classic anti-inflammatory interleukin, IL-4, decreases glucose uptake and lactate production. IL-4 stimulated microglia also downregulate Nitric Oxide (NO) and TNF-ɑ production as glucose is preferentially metabolised through oxidative phosphorylation (OxPhos) to increase ATP production. (B) The microglial inflammatory response is stimulated in a number of ways, such as infection (classically simulated with LPS and/or IFNɣ) or naturally through aging. These stressors have been shown to increase levels of the glucose transporter GLUT1 and thereby increase glycolytic and pentose phosphate pathway (PPP) flux, resulting in increased NADPH and ribose 5-phosphate availability to favor nucleotide synthesis versus ATP production. This push towards glycolysis and PPP is coupled with a decrease in mitochondrial respiration (OxPhos) and increased mitochondrial fission, resulting in increased mitochondrial number, but decreased mitochondrial size. Reactive oxygen species (ROS) production through NOX2 and Nitric Oxide (NO) production are increased in a manner dependent on glucose uptake. Prolonged increases in NO can inhibit oxidative phosphorylation further. (C) Interestingly, the inflammatory response is also activated following High Fat Diet (HFD) in hypothalamic microglia only in males. HFD in males diminished CX3CR1 signaling and led to an enhanced inflammatory response, while in females CXCL1 protein was increased, but overall CXC3R1 signaling remained relatively unaffected, and no increased inflammation was observed.
This figure is based on data presented in (Orihuela, McPherson, and Harry 2016; Jaber et al. 2017; Voloboueva et al. 2013; L. Wang et al. 2019; Dorfman et al. 2017)
