Abstract
Background and aim
In light of few established drug induced liver injury (DILI) registries, this study aims to evaluate the clinical spectrum and predictors of mortality and morbidity of hospitalized patients with suspected DILI.
Patients and methods
DILI cases were identified and categorized on basis of COIMS/RUCAM score and the exclusion of other liver diseases. Clinical and laboratory parameters were analyzed to identify the predictors of morbidity (prolonged hospital stay > 5 days) and mortality.
Results
Out of 462 patients, there were 264 (57.6%) males and the mean age of the cohort was 50.83 years (range: 20–94 years). DILI was classified as definite or highly probable in 31.1%, probable in 62.5%, and possible in 7.4% of cases. Pattern of liver injury was hepatocellular in 25.1%, cholestatic in 56.17%, and mixed in 18.72% of patients. Anti-tuberculosis drugs (ATDs) were found to be the most common category of drugs causing DILI, in 295 (63.9%) patients. Clinically, encephalopathy was present in 21.6% patients; other presenting symptoms included abdominal pain (57.1%), vomiting (57.1%), jaundice (54.1%) and pruritus (42.3%). In-hospital mortality was 26.5% and prolonged hospital stay (> 5 days) was observed in 35.93% of patients. Mortality was significantly greater in patients with encephalopathy, male gender, hepatocellular pattern of DILI, increased INR and use of ventilator support.
Conclusion
In our study, the most frequent cause of DILI in hospitalized patients was ATDs. More than a quarter of patients died during hospital stay. A close control of clinical and biochemical parameters are required to prevent and monitor DILI, especially in patients taking ATDs in our region.
Introduction
Drug induced liver injury (DILI) is defined as hepatotoxicity caused by various medications, herbs, or other xenobiotics, subsequently leading to abnormalities in liver tests or liver dysfunction with the reasonable exclusion of other etiologies [1]. Specific laboratory criteria are utilized to identify DILI: generally, a 3–5 times elevation of liver enzymes, namely transaminases (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]), alkaline phosphatase (ALP), or bilirubin, above their upper limit of normal (ULN) is required [2]. Altogether, in excess of a thousand medicines and chemicals have been implicated in drug induced liver injury [3, 4].
In the United States, DILI accounts for nearly 10% of the total cases of acute hepatitis, 5% of all hospital admissions, and 50% of all cases of acute liver failures [5]. DILI carries a mortality rate of approximately 10% [3–5]. It is the premier reason for drug withdrawal by the Food and Drug Administration (FDA) in the United States [5, 6].
The wide spectrum of clinical symptomatology, non-availability of specific diagnostic markers and lack of standardization between studies performed to date make it difficult to establish causality to a particular drug. Causal association to a specific drug is not a straightforward matter, as it heavilydepends on exclusion of other causes (notably viral and autoimmune hepatitis) and temporal relationship of the drug to the derangement in patient’s liver function tests (LFTs) [7]. As a result, sometimes certain scoring systems such as Roussel Uclaf Causality Assessment Method (RUCAM) [8], are used to assess the probability of association. The RUCAM system is a means of assigning points for clinical, biochemical, serologic and radiologic features of liver injury which gives an overall assessment score which reflects the likelihood that the hepatic injury is due to a specific medication [9].
Annual incidence of DILI ranges from 1.3 to 19 per 100,000 in various databases, depending on the country of origin, type of data and method of obtaining information [10–12]. The largest drug category responsible for DILI is antimicrobials, led by amoxicillin-clavulanate[13, 14]. Amongst antibiotics, ATDs are another major group associated with DILI especially in the developing world. Approximately 5.3% of all the cases in the United States DILI Network (US DILIN) were reported due to isoniazid (second only to amoxicillin-clavulanate) likewise 7% of the cases in the Spanish DILI Registry were due to isoniazid alone or in combination with other drugs [13, 14]. Other common drug groups include non-steroidal anti-inflammatory drugs (NSAIDs) [10, 14], herbal and dietary supplements (HDS) [15] and rarely statins [13, 16, 17].
A growing concern for pharmaceutical industry regarding drug development is hepatotoxicity induced by the newer molecular targeted agents (MTAs) which are increasingly being used in oncology. A third of patients treated with a protein kinase inhibitor experience liver injury, with pazopanib, sunitinib and regorafenib identified as the potentially lethal agents [18]. Similarly, 10% of patients treated with immune checkpoint inhibitors, such as ipilimumab, are susceptible to DILI [17]. Additionally, the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) gefitinib is associated with 18.5% frequency of hepatotoxicity. It has resulted in casualties as well [19].
In many countries, DILI registries have been set up which record every DILI case with a formal causality determination process, providing in-depth information about the types of drugs that cause DILI, the pattern of injury and the risk of mortality and morbidity. There are few established DILI registries in the region, and no centralized, national registry. This study aims to provide an analysis of clinical presentation and outcome of patients admitted with the discharge diagnosis of DILI from Pakistan.
Patients and methods
Ethics clearance
This study was reviewed and approved by ethics review committee of Aga Khan University (ERC-AKU).
Study design
A retrospective cross-sectional study.
Study setting and population
Patients admitted at Aga Khan University Hospital Karachi Pakistan, from January 2010 through December 2016, and discharged with a diagnosis of DILI, were recruited. The course of their hospital stay was reviewed through the medical record system.
Inclusion criteria
Patients with suspected diagnosis of DILI with clear documentation of the possible drug implicated were included.
Exclusion criteria
Patients with known or suspected acetaminophen toxicity, history of bone marrow or liver transplantation before the liver injury event, history of malignancy of liver and metastasis to liver, underlying hepatitis C virus (HCV), hepatitis B virus (HBV), or nonalcoholic fatty liver disease were all excluded alongside cases with other types of underlying chronic liver disease.
Criteria for diagnosis of DILI
The diagnosis of DILI and the causal relationship between liver injury event and implicated drugs were evaluated in a formal and standardized fashion by using a causality instrument: Roussel Uclaf Causality Assessment Method (RUCAM) [9]. Points were awarded for seven components comprising of the following: time to onset of the injury following start of the drug, subsequent course of the injury after stopping the drug, specific risk factors (age, alcohol use, pregnancy), use of other medications with a potential for liver injury, exclusion of other causes of liver disease, known potential for hepatotoxicity of the implicated drug and response to re-challenge. The RUCAM provides a semi-quantitative evaluation of causality by assigning −3 to +3 points to each of the aforementioned seven components. Based on the final score, a causal relationship between the implicated agent and the liver injury event was categorized as highly probable (>8), probable (6–8), possible (3–5), unlikely (1 or 2), or excluded (<0).
Criteria for assessment of clinical patterns of liver injury
According to the Council for International Organizations of Medical Sciences (CIOMS) criteria, DILI is classified as hepatocellular, cholestatic or mixed based on its R-value [9]. The R-value is defined as the serum ALT/ULN divided by the serum ALP/ULN ratio; R-values > 5 were classified as hepatocellular, < 2 as cholestatic and 2–5 as mixed injury [20].
Criteria for severity assessment
The severity assessment was done according to the Chinese guidelines for the diagnosis and treatment of DILI in 2015 [21]. The severity was scored as follows:
Mild: serum enzyme elevations with total bilirubin (TBil) < 2.5 × ULN and International Normalization Ratio (INR) < 1.5.
Moderate: serum enzyme elevations and TBil ≥ 2.5 × ULN or an INR ≥ 1.5.
Severe: serum enzyme elevations and TBil ≥ 5 × ULN with or without an INR ≥ 1.5.
Acute liver failure: serum enzyme elevations and TBil ≥ 10 × ULN or a daily elevation of TBil ≥ 17.1 μmol/L, an INR ≥ 2.0 and signs of hepatic or other organ failure related to DILI.
Assessment of patient morbidity and mortality
In-hospital morbidity was quantified in terms of prolonged hospital stay, defined as hospital stay for more than 5 days. Predictors of mortality and morbidity were assessed by considering patients’ clinical and laboratory parameters including liver synthetic functions (prothrombin time and serum albumin).
Statistical analysis
The statistical analysis was conducted by using the Statistical Package for Social Science (SPSS) (Release 19.0 standard version, copyright © SPSS). A descriptive analysis was performed and results are presented as mean ± standard deviation for quantitative variables and numbers (percentages) for qualitative variables. To analyze the risk factors for poor outcome, the categorical variables were evaluated using the chi-square test while the means were compared by Student t-test. Factors predicting prolonged hospital stay were analyzed by multivariate logistic regression analysis. To establish statistical significance, p value <0.05 was considered significant.
Compliance with ethical requirements
The study was undertaken upon receiving approval from Ethics Review Committee (ERC). Requirement for informed consent was waived by ERC. After completion of data collection by the authors, information was made anonymous for the statistician to proceed with data analysis.
Results
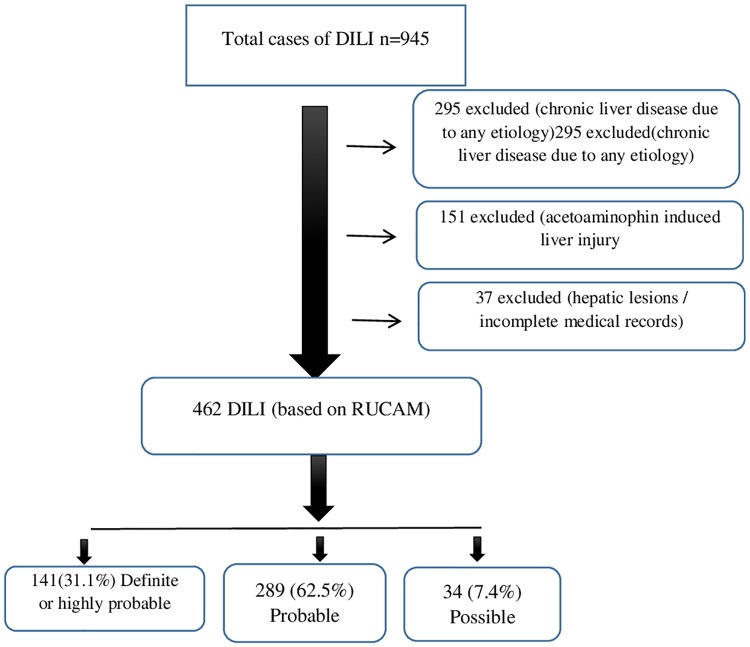
A total of 462 DILI cases were identified (Fig 1), out of which 264 (57.6%) patients were male with a mean age of 50.83 (range: 20–94). By using the RUCAM model for drug causality assessment, DILI was classified as definite or highly probable in 141 (31.1%), probable in 289 (62.5%) and possible in 34 (7.4%) cases.
Fig 1. Flow diagram showing selection DILI cases.
Pattern of liver injury
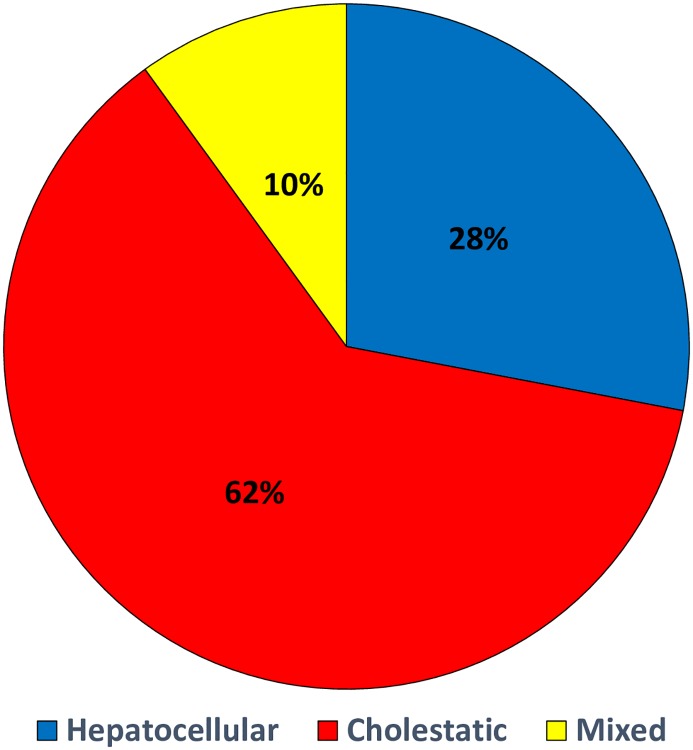
Pattern of liver injury was hepatocellular in 116 (25.1%), cholestatic in 260 (56.17%) and mixed in 86 (8.72%) patients with a discharge diagnosis of DILI (Fig 2).
Fig 2. Type of drug induce liver injury (n = 462).
Severity of liver injury
The severity of liver injury was found to be mild in 204 (44%), moderate in 78 (16.8%), and severe in 54 (13.8%) patients, while 116 (25.1%) cases were seen to have had liver failure due to drug intake. Mortality was significantly high in patients with liver failure (p value = 0.006). Table 1.
Table 1. Severity of DILI and relationship of age and gender with mortality (n = 462).
| Mild n = 204(44.2%) | Moderate n = 78(16.9%) | Severe n = 64(13.9%) | ACLF n = 116(25.1%) | p value | |
|---|---|---|---|---|---|
| Mortality | |||||
| Yes | 42(20.6) | 20(25.6) | 16(25) | 44(38.6) | 0.006 |
| No | 162(79.4) | 58(74.4) | 48(75) | 70(61.4) | |
| Age | |||||
| ≤35 years | 46(22.5) | 16(20.5) | 12(18.8) | 28(24.1) | 0.19 |
| 36–45 | 36(17.6) | 14(17.9) | 8(12.5) | 20(17.2) | |
| 46–55 | 40(19.6) | 10(12.8) | 12(18.8) | 26(22.4) | |
| 55–65 | 40(19.6) | 18(23.1) | 12(18.8) | 30(25.9) | |
| >65 yrs | 42(20.6) | 20(25.6) | 20(31.3) | 12(10.3) | |
| Gender | |||||
| Male | 102(50) | 52(66.7) | 50(78.1) | 62(53.4) | <0.001 |
| Female | 102(50) | 26(33.5) | 14(21.9) | 54(46.6) |
Presenting features of patients with DILI
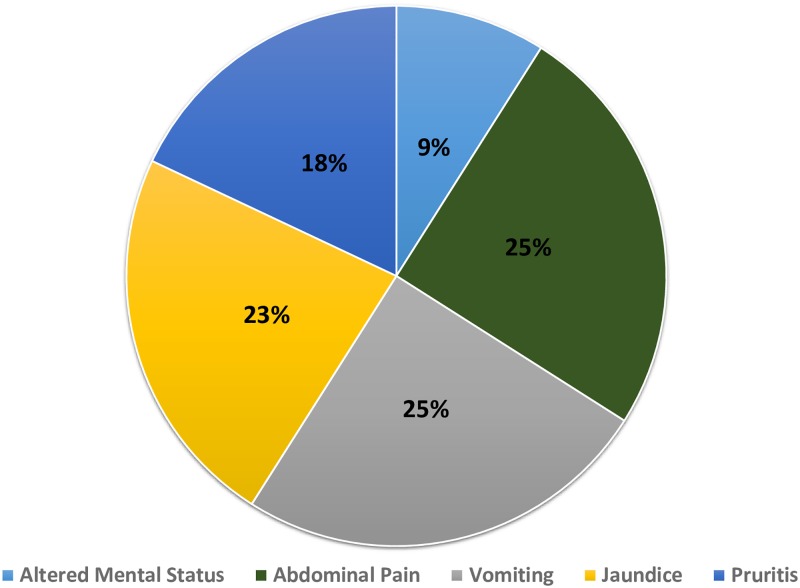
Encephalopathy was present in 98 (21.6%) patients on the day of hospital admission while patients who presented with abdominal pain, vomiting, jaundice and pruritus were in the following order: 57.1%, 57.1%, 54.1%, and 42.3% (Fig 3).
Fig 3. Presenting features of patients with drug induced liver injury (n = 462).
Furthermore, mean total bilirubin levels, ALT and AP levels were 5.37mg/dl (range: 0.20–79.1), 358.65(range: 7–8938) IU/L and 168.68(range: 32–1040) IU/L respectively.
Drug categories causing DILI
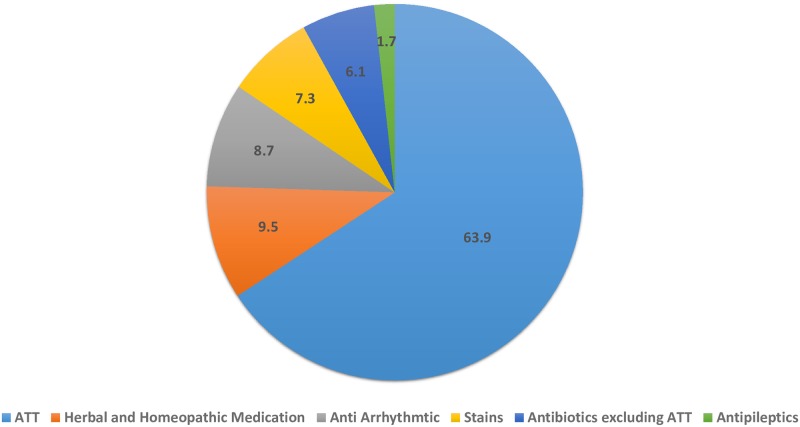
The top three causes of DILI in our study were anti-tuberculosis drugs (ATDs) followed by homeopathic or herbal medications and antiarrhythmic drugs. Patients taking ATDs in combination with different medications are listed (Table 2).
Table 2. Patients on anti-tuberculosis drugs.
| Combination of drugs | Number of patients n = 295 (%) |
|---|---|
| ATDs alone | 182(61.6) |
| ATDs with NSAID | 26(8.8) |
| ATDs with antibiotic | 83(28.1) |
| ATDs with antiepileptics | 4(1.3) |
Other drugs implicated are displayed on Fig 4.
Fig 4. Drug categories causing drug induced liver injury (n = 462).
Predictors of mortality and morbidity
All patients were managed as per standards of care, essentially supportive treatment was administered. A subset of patients with severe DILI received N- acetyl cysteine, and a subset of patients required ventilator support. In-hospital mortality was 122 out of 462 (26.5%) and morbidity (quantified as prolonged hospital stay more than 5 days) was observed in 214 out of 462 (35.93%) patients. None of the patients underwent a liver transplant due to non-availability of the facility at our institution and in the city during that time period.
On multivariate analysis, mortality was significantly greater in patients with encephalopathy, male gender, hepatocellular pattern of DILI, increased INR (>1.5), acute liver failure and patients who were on ventilator support in ICU. Table 3.
Table 3. Predictors of mortality of drug induced liver injury (n = 462).
| Patient characteristics | Death (n = 122) | Survival (n = 340) | p value |
|---|---|---|---|
| Age, in years | 53.3 ± 15.1 | 49.8 ± 17.0 | 0.03 |
| Gender | |||
| Male | 80(65.6) | 184(54.4) | 0.03 |
| Female | 42(34.4) | 154(45.6) | |
| DM | 30(24.6) | 70(20.7) | 0.22 |
| Dyslipidemia | 50(41) | 108(32) | 0.07 |
| ATT | 70(57.4) | 224(66.7) | 0.08 |
| Antibiotic | 8(6.6) | 20(5.9) | 0.80 |
| Antiepileptic | 0 | 8(2.4) | 0.08 |
| Antifungal | 10(8.2) | 16(4.7) | 0.15 |
| Atorvastatin | 10(8.2) | 22(6.5) | 0.57 |
| Chemotherapy | 10(8.2) | 4(1.2) | <0.001 |
| Herbal | 10(8.2) | 32(9.5) | 0.63 |
| Antimalarials | 6(5.6) | 4(1.4) | 0.02 |
| Digoxin | 0 | 12(4.3) | 0.006 |
| Antidepressants | 2(2.0) | 14(5.0) | 0.17 |
| Altered Mental Status | 46(37.7) | 52(15.4) | <0.001 |
| Jaundice | 74(60.7) | 174(51.5) | 0.08 |
| Pruritus | 78(63.9) | 184(54.4) | 0.06 |
| Abdominal pain | 120(98.4) | 326(96.4) | 0.26 |
| N acetyl cysteine | 38(31.1) | 54(16) | <0.001 |
| Hospital stay in days | 10.7 ± 10.9 | 6.7 ± 6.5 | <0.001 |
| Intubation | 32(38.1) | 18(7.0) | <0.001 |
| TB | 6.9 ± 12.8 | 4.8 ± 7.0 | 0.09 |
| IB | 2.5 ± 5.7 | 1.08 ± 1.4 | 0.007 |
| PT | 19.4 ± 13.0 | 15.0 ± 7.0 | <0.001 |
| INR | 1.93 ± 1.3 | 1.45 ± 0.65 | <0.001 |
| AP | 202.7 ± 183.1 | 165.3 ± 121.2 | 0.03 |
Likewise, prolonged hospital stay (duration of >5 days) was associated with female gender, increased ALT, AST aspartate aminotransferase levels, use of ventilator support and mixed pattern of DILI. Table 4.
Table 4. Predictors of prolonged hospital stay (>5 days) of patients with DILI (n = 462).
| Patient characteristics | < 5 days (n = 248) | >5 days (n = 214) | p value |
|---|---|---|---|
| Age, in years | 50.2 ± 16.8 | 51.3 ± 16.4 | 0.45 |
| Gender | |||
| Male | 154(62.1) | 112(52.6) | 0.03 |
| Female | 94(37.9) | 101(47.4) | |
| DM | 48(19.4) | 31(14.6) | 0.17 |
| Dyslipidemia | 51(20.6) | 50(23.5) | 0.45 |
| ATT | 43(76.8) | 37(58.7) | 0.03 |
| Antibiotics | 4(7.0) | 4(6.3) | 0.88 |
| Antiepileptics | 2(3.5) | 1(1.6) | 0.50 |
| Antifungal | 1(1.8) | 3(4.8) | 0.35 |
| Amiodarone | 5(8.8) | 5(7.9) | 0.86 |
| Statins | 6(10.5) | 5(7.9) | 0.62 |
| Chemotherapy | 1(1.8) | 3(4.8) | 0.38 |
| Herbal | 4(7.0) | 7(11.1) | 0.45 |
| Antimalarials | 0 | 2(4.8) | 0.49 |
| Mortality | 27(22.1) | 34(32.7) | 0.07 |
| N acetyl cysteine | 21(17.4) | 26(25) | 0.16 |
| History of alcohol | 1(1.8) | 2(3.3) | 0.58 |
| Intubation | 7(12.5) | 15(25) | 0.08 |
| TB | 4.9 ± 9.2 | 6.0 ± 11.4 | 0.25 |
| DB | 2.6 ± 2.3 | 5.1 ± 8.6 | 0.03 |
| IB | 1.11 ± 1.2 | 2.72 ± 5.9 | 0.04 |
| GGT | 151.3 ± 138.0 | 129.8 ± 149.9 | 0.11 |
| SGPT | 308.6 ± 630.9 | 439.1 ± 1093.0 | 0.12 |
| AP | 177.0 ± 125.8 | 165.7 ± 135.7 | 0.35 |
| SGOT | 400.1 ± 765.9 | 695.8 ± 1416.5 | 0.17 |
| R ratio | 9.6 ± 23.8 | 11.6 ± 21.7 | 0.34 |
Discussion
Drug induced liver injury is the most under-recognized and under-reported cause of liver injury, ultimately leading to underestimation of its burden. The present study analyzes hospitalized patients suffering from drug induced liver injury who were admitted in a tertiary care center in Pakistan, over a seven-year period. This is a large data set related to DILI from a developing country from where there is paucity of such kind of information.
One important finding from this study is that the order and frequency of drugs associated with DILI is different from the list provided in the report from the Drug Induced Liver Injury Network (DILIN) and the Spanish Registry [13, 14]. These studies showed that amoxicillin-clavulanate was the most common causative agent amongst the antimicrobials. A recently published review found 9 of the top 10 causes of DILI to be antibiotics; this is a measure of their hepatotoxic potential, as well as the common use and duration of treatment with these drugs [20, 22].
We found ATDs to be the most commonly implicated drug with approximately 64% of cases that were reviewed having received ATDs. This likely reflects the differences in the epidemiology of infectious diseases and corresponded to numbers observed by other studies from this region [23]. ATDs were followed by homeopathic and herbal medications with 9% of cases having received it, similar to other prior studies [24]. After ATDs, the category of drugs most frequently implicated in DILI was homeopathic and herbal medications, with a frequency within a range provided in prior studies from regions with a history of common consumption [24].
More than 20% of patients in our series had encephalopathy accounting for fulminant or acute liver failure at the time of presentation in the hospital. Conversely, the Spanish registry reported very low number of patients with fulminant hepatic failure with 11 out of 439 cases being classified as such [14, 25]. The high prevalence of encephalopathy in our study can be attributed to a delay in presentation to the hospital with very little knowledge about the drug being a cause of liver injury. Additionally, our center is one of the main tertiary care hospitals in Pakistan that receives an increasing number of complicated referrals: this may have resulted in more serious clinical presentations and contributed to greater DILI cases arising due to ATDs.
Another noteworthy observation deduced from our study is the fact that more than a quarter of hospitalized patients with DILI died while in the hospital. The mortality rate in our study appeared significantly high compared to that observed in several other studies, which ranges from 10 to 17.3% [10, 13, 26, and 27]. This difference in mortality is perhaps due to the fact that our series of DILI is for hospitalized patients which are expected to be more severely ill. Another factor for high mortality in our study could be the fact that ATDs was the leading cause of DILI as it has been observed in an Indian study that mortality in DILI patients on ATDs was significantly high compared to those not taking ATDs: 21.5% vs. 11.4% respectively (p = 0.02)[28]. Lack of facilities for liver transplantation could be another reason for high mortality in our series.
Very few studies have reported predictors of outcome for DILI which include hepatocellular damage, high bilirubin and female sex, as described by the US DILI network [15]. The Spanish registry and a Swedish study have described the hepatocellular pattern of damage as the most common form of liver injury associated with high incidence of liver transplantation or death if patient with jaundice [14, 27].
In a Chinese study, ATDs were found to be the primary etiological factor for fatal DILI. Additionally, the same study also identified that hepatic encephalopathy, ascites, jaundice, alcohol abuse and direct bilirubin levels were associated with the death of DILI patients [29]. Likewise in an Indian study, high-MELD score or a combination of ascites, encephalopathy, high bilirubin, prothrombin time, and leukocyte count were identified as predictors of mortality [28]. In our study, we also observed that mortality was significantly greater in patients with encephalopathy, male gender, hepatocellular pattern of DILI, increased INR (>1.5) and patients on ventilator support.
Limitations of the present study include a retrospective study design and a sample population based in a single tertiary care center setting. Non-availability of transplantation facility for ultimate treatment of patients restricted us from reviewing the outcomes in such patients in detail.
Conclusion
In the present study, ATDs was seen to be the most frequent cause of DILI in hospitalized patients. More than a quarter of patients died during hospital stay. As a result, care among physicians is required while prescribing potentially hepatotoxic agents. A close control of clinical and biochemical parameters is required while prescribing potentially hepatotoxic agents, especially ATDs in our region. Additionally, efforts at the national level should be undertaken to create greater public awareness about DILI especially while using ATDs.
Supporting information
(RTF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research project was fully sponsored by Ferozsons Private Limited, Drug Induced Liver Injury Project (DILI-N); GC # CON000000000431. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Drug Induced Liver Injury N. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135(6): 1924–1934.e1924 10.1053/j.gastro.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeff LB. Drug-induced liver injury is a major risk for new drugs. J Dig Dis 2015; 33(4): 458–463 10.1159/000374089 [DOI] [PubMed] [Google Scholar]
- 3.Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinosporacordifolia (Willd.)Hook.f. and Thoms.(Guduchi)-validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res 2010; 1(2): 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porceddu M, Buron N, Roussel Cl, Labbe G, Fromenty B, Borgne-Sanchez A. Prediction of liver injury induced by chemicals in human with a multiparametric assay on isolated mouse liver mitochondria. ToxicolSci 2012; 129(2): 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity: a review. J Appl Pharm Sci 2012; 2(5): 233–243. [Google Scholar]
- 6.Ostapowicz G, Fontana RJ, SchiÃdt FV, Larson A, Davern TJ, Han SHB, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137(12): 947–954 10.7326/0003-4819-137-12-200212170-00007 ] [DOI] [PubMed] [Google Scholar]
- 7.Ahmad J, Odin JA. Epidemiology and genetic risk factors of drug hepatotoxicity. Clinics in liver disease. 2017;21(1):55–72 10.1016/j.cld.2016.08.004 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal VK, McHutchison JG, Hoofnagle JH, Network DILI. Important elements for the diagnosis of drug-induced liver injury. ClinGastroenterolHepatol 2010; 8(5): 463–470 10.1016/j.cgh.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. ClinEpidemiol 1993; 46(11): 1323–1330 10.1016/0895-4356(93)90101-6 ] [DOI] [PubMed] [Google Scholar]
- 10.Bjornsson ES, Bergmann OM, BjÃrnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144(7): 1419–1425.e1413 10.1053/j.gastro.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Sgro C, Clinard Fo, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatol 2002; 36(2): 451–455 10.1053/jhep.2002.34857 [DOI] [PubMed] [Google Scholar]
- 12.Ruigomez A, Brauer R, RodrÃguez LAG, Huerta C, Requena G, Gil M, et al. Ascertainment of acute liver injury in two European primary care databases.ClinPharmacol 2014; 70(10): 1227–1235 10.1007/s00228-014-1721-y [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015; 148(7): 1340–1352.e1347 10.1053/j.gastro.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade RlJ, Lucena MI, FernÃndez MC, Pelaez G, Pachkoria K, García-Ruiz E,et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129(2): 512–521 10.1016/j.gastro.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Navarro V. J., Barnhart H., Bonkovsky H. L., Davern T., Fontana R. J., Grant L., R K. et al. 2014. "Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network." Hepatology 60 (4):1399–408. 10.1002/hep.27317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 2012; 56(2): 374–380 10.1016/j.jhep.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 17.Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization.Am J Med 2005; 118(6): 618–624 10.1016/j.amjmed.2005.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL,et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 2017; 66(6): 1154–1164 10.1136/gutjnl-2016-313369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015; 88(1): 74–79 10.1016/j.lungcan.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 20.Pang L, Yang W, Hou F. Features and outcomes from a retrospective study of 570 hospitalized Chinese patients with drug-induced liver injury. Clinics and research in hepatology and gastroenterology. 2018;42(1):48–56 10.1016/j.clinre.2017.08.003 ] [DOI] [PubMed] [Google Scholar]
- 21.Yu Y-c, Mao Y-m, Chen C-w, Chen J-j, Chen J, Cong W-m, et al. guidelines for the diagnosis and treatment of drug-induced liver injury.HepatolInt 2017; 11(3): 221–241 10.1007/s12072-017-9793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoofnagle Jay H., and Björnsson Einar S. 2019. "Drug-Induced Liver Injury—Types and Phenotypes." New England Journal of Medicine 381 (3):264–273. 10.1056/NEJMra1816149 23 Rathi C, Pipaliya N, Patel R, Ingle M, Phadke A, Sawant P. Drug induced liver injury at a tertiary hospital in India: Etiology, clinical features and predictors of mortality. Annals of hepatol 2017; 16(3): 442–450 [28425415 10.5604/16652681.1235488]. [DOI] [PubMed] [Google Scholar]
- 23.Almdal TP, Sorensen TIA. Incidence of parenchymal liver diseases in Denmark, 1981 to 1985: analysis of hospitalization registry data. Hepatol 1991; 13(4): 650–655 [PubMed] [Google Scholar]
- 24.Jing J, Teschke R. Traditional Chinese medicine and herb-induced liver injury: comparison with drug-induced liver injury. J ClinTranslHepatol 2018; 6(1): 57 10.14218/JCTH.2017.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase:unified list based on international collaborative work. Drug safety 2010; 33(6): 503–522 10.2165/11535340-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 26.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014; 109(7): 950 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 27.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatol 2005; 42(2): 481–489 10.1002/hep.20800 [DOI] [PubMed] [Google Scholar]
- 28.Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010. November; 105(11):2396–404 10.1038/ajg.2010.287 [DOI] [PubMed] [Google Scholar]
- 29.Li B, Wang Z, Fang J-J, Xu C-Y, Chen W-X. Evaluation of prognostic markers in severe drug-induced liver disease.World J. Gastroenterol 2007; 13(4): 628 [DOI] [PMC free article] [PubMed] [Google Scholar]