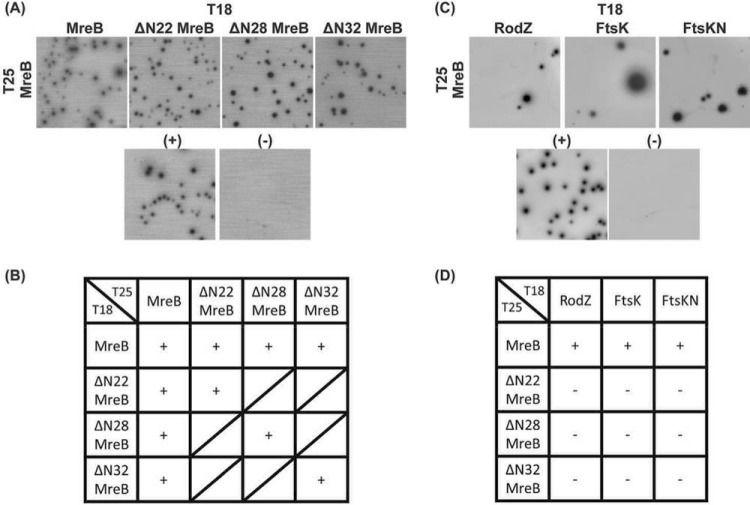
FIG 7.
BACTH assay to test chlamydial protein-protein interactions of full-length and truncated MreB with divisome components. E. coli DHT1 (Δcya) was cotransformed with vectors encoding the indicated genes fused to the T25 and T18 catalytic domains of the Bordetella pertussis adenylate cyclase. Transformants were plated on M63 minimal medium plates containing 50 μg/ml ampicillin, 25 μg/ml kanamycin, 0.5 mM IPTG, 40 μg/ml X-Gal, 0.04% Casamino Acids, and 0.2% maltose. The plates were incubated at 30°C for 5 to 7 days. (A and B) BACTH assay of the interaction between full-length and truncated chlamydial MreBs. (C and D) BACTH assay of the interaction between full-length or truncated chlamydial MreBs and previously described chlamydial cell division components FtsK, the N-terminal domain of FtsK (FtsKN), and RodZ. A positive control [(+)] is the interaction between T25-Zip and T18-Zip, the GCN4 leucine zipper motif, and a negative control [(−)] is the lack of interaction between T25 and the mixture of T18-MreB and T18-ΔN22 MreB. These tests were performed a minimum of two times. +, interaction; −, no interaction; ∕, not tested.