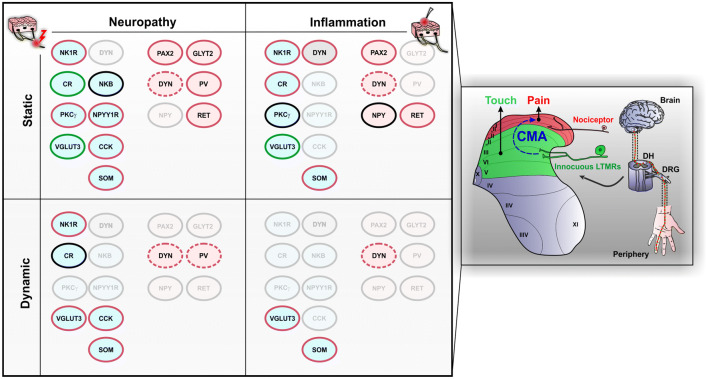
Fig. 3.
Populations of dorsal horn neurons involved in cutaneous mechanical allodynia. Schematic representation on the right illustrates the segregation of touch and pain in the spinal cord dorsal horn. In physiological conditions, non-nociceptive sensory neurons (LTMRs) innervate the deep DH (green), whereas nociceptive fibers terminate in the superficial laminae (red). During cutaneous mechanical allodynia (CMA), touch induces pain through activation of a polysynaptic circuit (blue) that connects the non-nociceptive deep DH to the pain‐related superficial layers. Populations of neurons that have been implicated in CMA are indicated on the left part of the figure, with distinctions between static/dynamic and neuropathic/inflammatory CMA. Populations of neurons that are mostly excitatory are displayed in light blue ellipses and those that are mostly inhibitory neurons are displayed in light pink ellipses. Lamina I neurons expressing dynorphin (DYN) include both inhibitory and excitatory cells and are displayed as gray ellipses. Populations of neurons that have not been tested for the respective conditions are transparent. Outlines in red indicate that the population has been implicated in the expression of CMA, in green indicates that the population is engaged but not required for CMA and in black indicates that the population is dispensable for CMA. Dotted outlines indicate that the requirement of the population for CMA is unclear. DH dorsal horn, DRG dorsal root ganglion, LTMR low‐threshold mechanoreceptor, NK1R neurokinin 1 receptor, CR calretinin, PKCγ gamma isoform of protein kinase C, VGLUT3 vesicular glutamate transporter 3, DYN dynorphin, NKB neurokinin B, NPYY1R neuropeptide Y Y1 receptor, CCK cholecystokinin, SOM somatostatin, PAX2 paired box gene 2, NPY neuropeptide Y, GLYT2 glycine transporter 2, PV parvalbumin, RET receptor tyrosine kinase