Significance
We report that CD40 activation can provide an immunological “missing link” by priming T cells and synergizing with immune checkpoint blockade in a highly refractory pancreas cancer genetic mouse model. The underlying mechanism is mediated by CD40 ligation, representing an alternative molecular pathway distinct from pattern recognition receptor stimulation that connects dendritic cell activation to adaptive immunity, enabling T cell priming that is otherwise unachievable in pancreatic cancer.
Keywords: CD40, T cell, dendritic cell, pancreatic cancer
Abstract
Innate immune receptors such as toll-like receptors (TLRs) provide critical molecular links between innate cells and adaptive immune responses. Here, we studied the CD40 pathway as an alternative bridge between dendritic cells (DCs) and adaptive immunity in cancer. Using an experimental design free of chemo- or radiotherapy, we found CD40 activation with agonistic antibodies (⍺CD40) produced complete tumor regressions in a therapy-resistant pancreas cancer model, but only when combined with immune checkpoint blockade (ICB). This effect, unachievable with ICB alone, was independent of TLR, STING, or IFNAR pathways. Mechanistically, αCD40/ICB primed durable T cell responses, and efficacy required DCs and host expression of CD40. Moreover, ICB drove optimal generation of polyfunctional T cells in this “cold” tumor model, instead of rescuing T cell exhaustion. Thus, immunostimulation via αCD40 is sufficient to synergize with ICB for priming. Clinically, combination αCD40/ICB may extend efficacy in patients with “cold” and checkpoint-refractory tumors.
The discovery and elucidation of pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs, respectively) revolutionized the understanding of mechanisms by which activation of innate immunity converts to the triggering of robust adaptive responses (1). At a cellular level, these molecular ligands connect dendritic cells (DCs) and myeloid cells with B and T lymphocytes that respond with memory to insults from invasive pathogens or damaged tissue. Such ligands also explain the molecular basis of many vaccine adjuvants, once termed “immunology’s dirty little secret” by a pioneer in this very field (2). Indeed, the toll-like receptor (TLR) agonist imiquimod (which binds TLR7) is approved by the Food and Drug Administration for basal cell carcinoma (3) and shows some efficacy in vulvar intraepithelial neoplasia (4), while a TLR3 agonist showed success in a small clinical trial of patients with melanoma (5). For many aggressive tumors, however, it has been difficult to translate the use of pattern recognition receptor (PRR) ligands as effective therapy, despite major recent advances in cellular immunotherapy including immune checkpoint blockade (ICB) (6). For example, unmethylated CpG, which binds to TLR9 and activates antigen-presenting cells, failed to show efficacy sufficient to warrant regulatory approval when administered alone or as part of other cancer therapies (7–9).
We hypothesize that CD40 represents a distinct and alternative pathway for bridging DC activation and adaptive immunity in cancer independently of certain PRRs that have been increasingly implicated in tumor immune signaling. As a member of the tumor necrosis factor (TNF) superfamily of receptors, CD40 is expressed by DCs, myeloid cells, and B cells—among others—where ligation results in cellular activation. Instead of a primitive signal via a PAMP or DAMP (10, 11), CD40 is activated by CD40 ligand (CD40L) primarily expressed on CD4+ helper T cells. As reviewed elsewhere (12, 13), CD40 activation enables DC licensing and maturation important for priming cytotoxic T cells. Agonist αCD40 monoclonal antibodies (mAbs) mimic CD40L in vivo and have been shown to enhance immunogenicity of cancer vaccines and trigger regressions of highly immunogenic tumors (14–17). We and others have shown that when added to TLR agonists, chemotherapy, or radiation therapy, αCD40 can drive T cell responses and tumor rejection (18–24) and synergizes with ICB (25, 26). Based on experiments with knockout (KO) or mutant mice, we found that signaling via TLRs, the inflammasome, STING, or type I interferon (IFN) receptors was not required for the efficacy of αCD40 and chemotherapy (22, 23). However, a role for chemotherapy or radiotherapy in triggering a DAMP response could not be excluded.
Here, we developed an effective treatment regimen with αCD40 and ICB (αPD-1/αCTLA-4) using a syngeneic mouse model based on the genetically engineered KrasLSL-G12D/+,Trp53LSL-R172H/+,Pdx1-Cre (KPC) model of pancreatic ductal adenocarcinoma (PDA) (25). Neither chemotherapy nor radiation was employed. Combination αCD40/ICB resulted in tumor regressions and immunological memory, but not αCD40 or ICB alone. We found that αCD40/ICB function independently of TLR, STING, and type I IFN pathways in this immunologically “cold” tumor, leading to T cell priming rather than reversal of T cell exhaustion.
Results
ICB synergizes with αCD40 to Cure Immunologically Cold Tumors in the Absence of Chemotherapy.
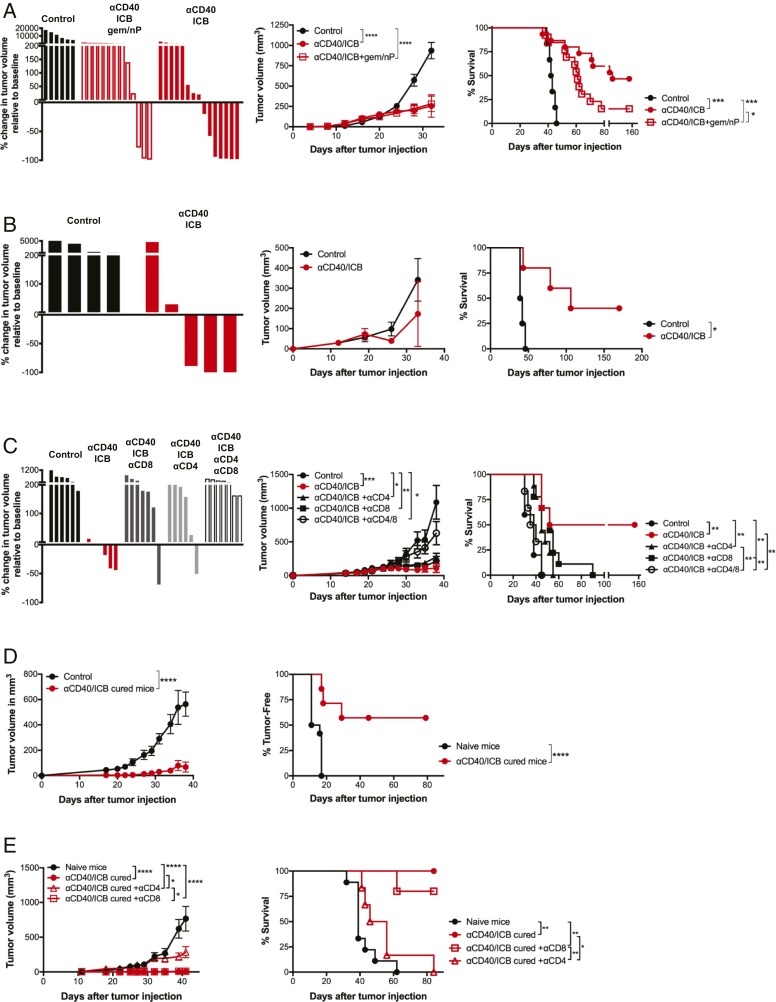
To discern the role of CD40 in immune activation, we adapted our tumor treatment model to eliminate confounding variables from chemotherapy or radiation. C57BL/6 mice were injected subcutaneously (s.c.) with a polyclonal PDA cell line (4662) that was generated from spontaneously arising KPC tumor (22) and then treated with αCD40 and dual ICB (αPD-1 and αCTLA-4) either with or without standard chemotherapy (gemcitabine, Gem; nab-paclitaxel, nP) (treatment schema are shown in SI Appendix, Fig. S1A) (22, 25, 26). As expected, αCD40/ICB combined with chemotherapy induced tumor regressions, significantly slowed tumor growth, and improved long-term survival of the mice compared to vehicle control-treated mice (tumor growth P < 0.0001 and survival P = 0.008, αCD40/ICB/Gem/nP vs. control; Fig. 1A) (22, 25, 26). However, the antitumor effect of αCD40/ICB treatment was more prominent, inducing a greater number of tumor regressions (7/15 αCD40/ICB vs. 3/13 αCD40/ICB/Gem/nP) and improving long-term survival (P = 0.035 αCD40/ICB compared to αCD40/ICB/Gem/nP; Fig. 1A). The synergistic effect of αCD40/ICB combination therapy required both αCD40 and ICB, as neither alone was able to induce regressions, slow tumor growth, or improve survival (SI Appendix, Fig. S1B).
Fig. 1.
αCD40 and checkpoint blockade drive T cell responses independently of chemotherapy in PDA. (A) s.c. PDA tumors were implanted in C57BL/6 mice and treated as in SI Appendix, Fig. S1A with αCD40/ICB +/− chemotherapy. (Left) Waterfall plot indicating percent change in tumor volume from beginning of therapy until 2 wk after therapy. (Center) Tumor growth kinetics. (Right) Overall survival. Data are representative of three experiments with n = 5 to 15 mice per group. (B) Orthotopic PDA tumors were implanted into the pancreas of C57BL/6 mice and treated as in A with either αCD40/ICB or isotype controls. Data are representative of three experiments with n = 5 to 8 mice per group. (C) C57BL/6 mice bearing s.c. PDA were treated as in A and depleted of either CD8+, CD4+, or both CD8+ and CD4+ T cells starting 1 d prior to the start of αCD40/ICB and continuing twice weekly throughout treatment. Data are representative of two experiments with n = 8 to 10 mice per group. (D) C57BL/6 mice treated as in A and cured of PDA tumors or naïve C57BL/6 mice were challenged with parental PDA and tumor growth and tumor-free survival were measured. For survival, each point represents when a single mouse died or was censored. (Left) Tumor growth kinetics. (Right) Overall survival. Data are representative of four experiments with n = 4 to 7 mice per group. (E) C57BL/6 mice treated as in A and cured of PDA tumors or naïve C57BL/6 mice were challenged with parental PDA. Some mice were depleted of CD4+ or CD8+ T cells beginning 2 d before tumor injection and continuing twice weekly for 5 wk as indicated. (Left) Tumor growth kinetics. (Right) Overall survival. Data are representative of two experiments with n = 4 to 6 mice per group. For waterfalls, each bar represents a single mouse. For growth curves, each point represents the average of n = 5 to 10 mice and error bars show the SEM. For survival curves, each dot represents the death or censoring of a single mouse. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
To ensure the efficacy of αCD40/ICB was not due the unique immune environment of the skin, we orthotopically injected 4662 cells into the pancreata of C57BL/6 mice. ICB is not sufficient to drive tumor regressions or long-term survival in orthotopic PDA-tumor bearing mice (SI Appendix, Fig. S1C), in concordance with our previous report (25). The combination of αCD40 and ICB again induced regressions in the majority of tumor-bearing mice, slowed tumor growth, and led to significantly improved long-term survival compared to control-treated mice (P = 0.011), even in the more immunosuppressive pancreatic tissue site (Fig. 1B).
To investigate whether both αPD-1 and αCTLA-4 were required for therapeutic efficacy, we tested αCD40 with either αPD-1 or αCTLA-4 and compared to the triple combination of αCD40 with dual ICB (both αPD-1 and αCTLA-4). In the s.c. tumor model, there were no statistically significant differences in rates of tumor regression, tumor growth, and long-term survival between αCD40/dual ICB and αCD40/single ICB (either αPD-1 or αCTLA-4) (SI Appendix, Fig. S1D). However, in the orthotopic model, the rates of tumor regression and long-term cure were significantly higher when αCD40 was combined with dual ICB as compared to αCD40 combined with either single checkpoint antibody alone (P = 0.013 αCD40/dual ICB vs. αCD40/single ICB; SI Appendix, Fig. S1E). As such, we decided to focus on the more therapeutically powerful regimen of both αPD-1 and αCTLA-4 in combination with αCD40 for mechanistic studies.
αCD40/ICB-Induced Cures Are T Cell Dependent.
To investigate the cellular mechanism of tumor regression with αCD40/ICB, mice were depleted of T cells with either αCD4, αCD8, or both, prior to receiving therapy. Tumor regressions to αCD40/ICB were rare and tumor growth inhibition was slightly reduced after T cell depletion (Fig. 1C). Depletion of either CD4+ T cells, CD8+ T cells, or both, significantly reduced long-term survival of mice after therapy (Fig. 1C). Given the contribution of T cells to mediating this primary tumor response, we then hypothesized that a protective memory T cell response against the tumor had also been generated. We found that the majority (57%) of mice cured by αCD40/ICB went on to reject a subsequent rechallenge s.c. with 4662 (P = 0.0002; Fig. 1D). To investigate the role of CD4+ and CD8+ T cells in the memory response, cured mice were depleted of either CD4+ or CD8+ T cells prior to rechallenge with 4662. Mice depleted of CD4+ T cells were unable to reject the second tumor, whereas mice depleted of CD8+ T cells remained protected against tumor rechallenge (P = 0.050 cured vs. cured + αCD4; Fig. 1E), indicating that long-term memory is primarily mediated by CD4+ T cells.
αCD40 and ICB Combination Therapy Is Dependent on Batf3+ DC and Host Expression of CD40, but TLR, STING, or IFNAR Pathways Are Dispensable.
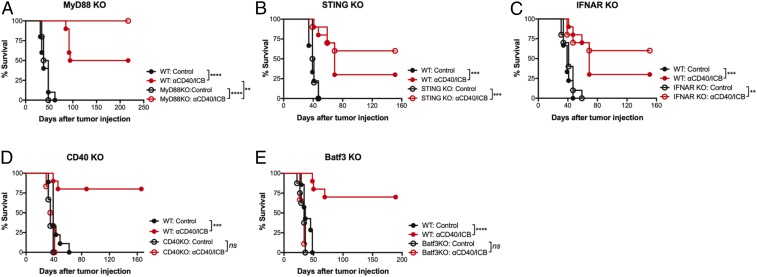
We have previously shown that αCD40, when combined with either chemotherapy or radiation therapy, stimulates T cell responses in the absence of multiple innate immune sensors (22, 23). Having established an effective treatment strategy without using chemotherapy or radiation, we then evaluated if αCD40/ICB was sufficient to prime a T cell response in the absence of either TLR or STING pathway stimulation. Mice lacking key components of the TLR and STING pathways—including MyD88, STING, and IFN alpha/beta receptor (IFNAR) KO mice—responded to αCD40/ICB with similar efficacy as wild-type mice (Fig. 2 A–C). Thus, αCD40 stimulation is sufficient to bypass innate immune sensor pathways and bridge innate and adaptive immunity in the absence of TLR and STING stimulation. However, when PDA tumors were implanted in CD40 KO mice, therapy was completely ineffective (Fig. 2D), confirming the requirement for host expression of CD40 for therapeutic efficacy. Moreover, therapeutic efficacy of αCD40/ICB was completely ablated in Batf3 KO mice [which lack cross-presenting CD103+ DCs (27)] (Fig. 2E). Therefore αCD40/ICB stimulation bypasses the need for innate immune activation via key PRRs but requires activation via the CD40 pathway and cross-presenting DCs for optimal T cell responses.
Fig. 2.
αCD40/ICB efficacy is independent of innate immune sensors but requires Batf3 and CD40 expression. (A–E) Wild-type mice or mice lacking the indicated gene were implanted with s.c. PDA and treated as in SI Appendix, Fig. S1A, as indicated. For waterfall plots, each bar represents a single mouse. For growth curves, each point represents the mean of n = 5 to 10 mice and error bars indicate the SEM. For survival curves, each dot represents the death or censoring of a single mouse. **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns indicates not significant.
CD40-Dependent Activation of Peripheral CD4+ and CD8+ T Cells after αCD40/ICB Therapy.
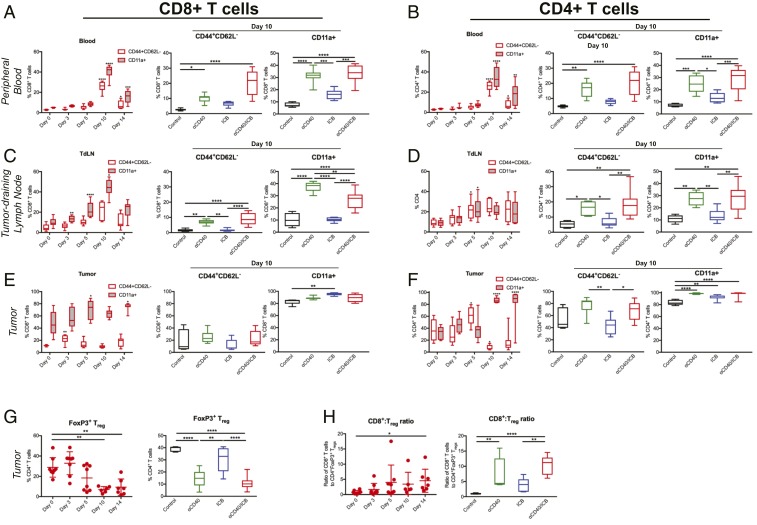
Given the requirement for Batf3 expression, we hypothesized that αCD40/ICB was promoting T cell priming and activation downstream of cross-presenting DCs. By 10 d after the initiation of therapy, there was a significant increase in the proportion of both CD8+ and CD4+ T cells displaying an activated effector phenotype (CD44+CD62L−) in the peripheral blood of treated mice (Fig. 3 A and B, Left), most strikingly in the blood of mice receiving either αCD40 or αCD40/ICB therapy (Fig. 3 A and B). CD11a expression [indicative of an activated polyclonal T cell population (28–30)] was found on a higher proportion of both CD4+ and CD8+ T cells after treatment, induced in a CD40-dependent manner (Fig. 3 A and B). This pattern of activation was also observed in both the CD44+CD62L− and the CD11a+ subsets of CD4+ and CD8+ T cells in the tumor-draining lymph node (TdLN) with a peak at the day-10 time point (Fig. 3 C and D), consistent with kinetics of a primary T cell response. Despite the spike in T cell activation markers in the periphery 10 d after the start of therapy, in the tumor site the proportion of CD44+CD62L− CD8+ T cells was relatively constant, whereas the subset of activated intratumoral CD4+ T cells was significantly reduced at day 10. In contrast, the CD11a+ subset of both CD8+ and CD4+ T cells was significantly increased at late time points (Fig. 3 E and F).
Fig. 3.
αCD40 promotes nonredundant systemic T cell activation independently of ICB treatment. C57BL/6 mice bearing s.c. PDA were treated as in SI Appendix, Fig. S1A. (A and B) Peripheral blood was analyzed at indicated time points for the proportion of CD44+CD62L− or CD11a+ cells among live CD45+CD3+ CD8+ (A) or CD4+ (B) cells. Graphs on right side depict comparison of all four treatment groups 10 d after the start of therapy. (C and D) TdLNs were harvested and analyzed at indicated time points for the proportion of CD44+CD62L− or CD11a+ cells among live CD45+CD3+ CD8+ (C) or CD4+ (D) cells. Graphs on right side depict comparison of all four treatment groups 10 d after the start of therapy. (E and F) Tumors were harvested and analyzed at indicated time points for the proportion of CD44+CD62L− or CD11a+ cells among live CD45+CD3+ CD8+ (E) or CD4+ (F) cells. Graphs on right side depict comparison of all four treatment groups 14 d after the start of therapy. (G) Tumors were harvested and analyzed at indicated time points for the proportion of FoxP3+ cells among live CD45+CD3+CD4+ cells, with all four groups depicted at day 14 after the start of treatment on the right. (H) The ratio of CD8+ T cells to FoxP3+ CD4+ T cells among live, CD45+CD3+ cells at indicated time points is shown with all four groups depicted at day 14 after the start of treatment on the right. For dot plots, each point represents a single mouse, the horizontal line indicates the mean, and error bars show the SD. For box and whisker plots, middle line indicates the median, extent of box indicates the interquartile range, and whiskers represent the 95% confidence interval of n = 5 to 10 mice/group, representative of n = 2 or 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, comparing each time point to the day 0 time point, before treatment was initiated, in the graphs on the right side of A–F, or as indicated by brackets in the middle and left graphs of A–F, as well as in G and H.
The modest changes in the activated intratumoral T cell subsets suggested other T cell subsets were also altered after treatment. Beginning as early as 48 h after αCD40 administration, αCD40/ICB decreased the proportion of CD4+ T cells that were FoxP3+ regulatory cells (Tregs) (Fig. 3G and SI Appendix, Fig. S2A). This effect was due solely to αCD40 with no significant Treg depletion from αPD1 or αCTLA-4 (Fig. 3 G, Right and SI Appendix, Fig. S2B) in the KPC model, unlike the Treg-depleting effect of αCTLA-4 (clone 9H10) in the B16 model (SI Appendix, Fig. S2B) (31). The reduction of Tregs in the tumor microenvironment (TME) after αCD40 +/− ICB improved the ratio of CD8+ T cells to Tregs in the tumor site (Fig. 3H), skewing the TME in favor of effector cells.
Combination αCD40/ICB Therapy Synergistically Enhances Functional T Cell Subsets.
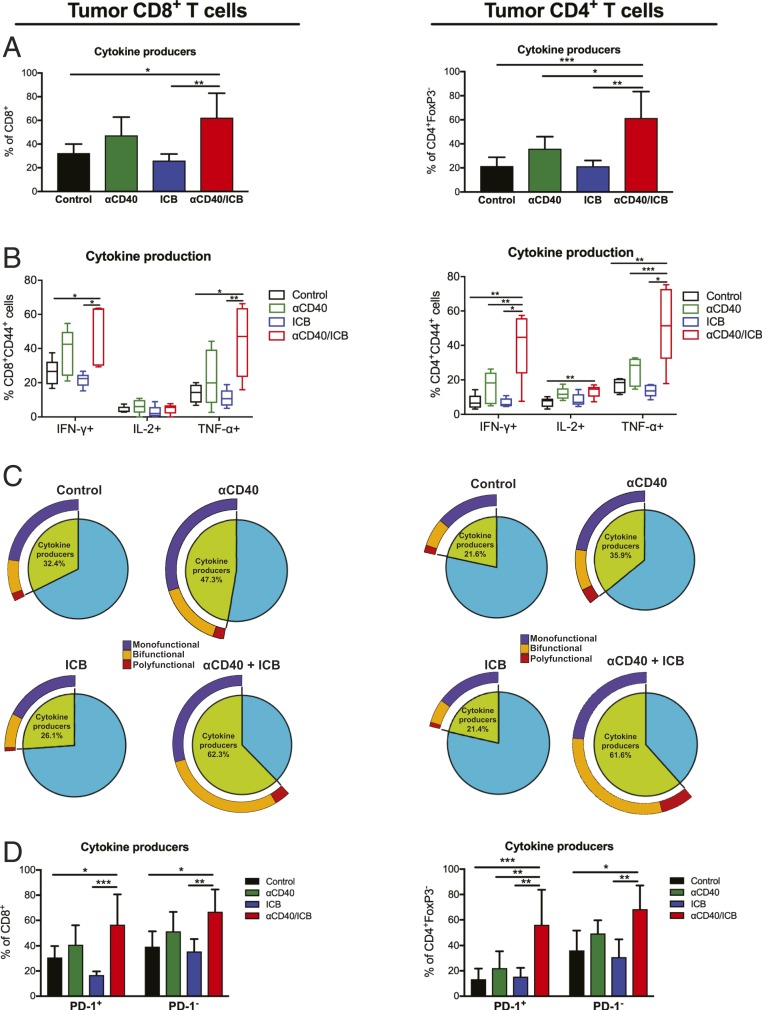
Given the alterations within the effector and suppressive T cell subsets in the TME after αCD40 treatment, we next investigated if the posttreatment T cell population was more functional. Prior to therapy, only 32% of CD8+ T cells and 22% of CD4+ T cells were cytokine producers in control-treated mice, generating IFN-γ, TNF-α, or interleukin (IL)-2 in response to ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin. Treatment with ICB or αCD40 alone was insufficient to increase cytokine production by either CD8+ or CD4+ T cells compared to control treatment (Fig. 4A). However, the combination of both αCD40/ICB significantly increased the proportion of both CD4+ and CD8+ T cells capable of producing cytokines. This was reflective of a significant increase in the proportions of CD4+ and CD8+ T cells generating IFN-γ or TNF-α after αCD40/ICB therapy compared to control treatment (or αCD40 or ICB alone) (Fig. 4B).
Fig. 4.
Functional PD-1+ and PD-1− T cell subsets are generated only after combination αCD40/ICB therapy. s.c. tumors were injected into wild-type C57BL/6 mice and treated as described in SI Appendix, Fig. S1A. Tumors were harvested 10 d after beginning therapy and incubated in PMA/ionomycin with Brefeldin A for 3 h before proceeding with extracellular and intracellular staining. (A) Graphs indicate the total percentage of live CD45+CD3+ CD8+ (Left) or CD4+FoxP3− (Right) cells expressing IFN-γ, TNF-⍺, and/or IL-2 (“cytokine producers”). (B) Graphs indicate the percentage of Live CD45+CD3+ CD8+ (Left) or CD4+FoxP3− (Right) cells that are IFN-γ+, TNF-⍺+, or IL-2+ among each indicated treatment group. (C) Pie chart representing the fraction of “cytokine producer” CD4+ or CD8+ T cells, with exploded ring indicating the proportion of cytokine producers generating one (“monofunctional”), or combinations of two (“bifunctional”) or more (“polyfunctional”), of the above cytokines with the indicated treatment. (D) Graphs indicate the total percentage of “cytokine producers” among Live CD45+CD3+ CD8+ (Left) or CD4+ (Right) cells, segregated by PD-1 status, after indicated treatment. For bar graphs, bars indicate mean and error bars show the SD. For box and whisker plots, horizontal line indicates the median, extent of box indicates the interquartile range, and whiskers represent the 95% confidence interval of n = 5 to 10 mice. For pie charts, indicated fractions are an average over n = 5 to 10 mice per group. Data are representative of two or three experiments with n = 5 to 10 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001.
To assess the quality of the functional T cell response, we compared the proportion of T cells producing one or more cytokines after therapeutic intervention. Treatment with αCD40 or combination αCD40/ICB therapy resulted in significant increases in the proportion of T cells capable of producing multiple cytokines (bifunctional or polyfunctional) compared to control treatment (P = 0.03 for CD8+ T cells and P = 0.0008 for CD4+ T cells; Fig. 4C). After combination αCD40/ICB therapy, 32.9 ± 18.2% SD of CD8+ and 37.5 ± 17.4% SD of CD4+ T cells produced at least two cytokines, threefold the proportion of multifunctional T cells from control-treated mice (9.6 ± 4.7% SD for CD8+ T cells, 7.8 ± 2.8% SD for CD4+ T cells; Fig. 4C).
Given the canonical view that ICB rescues exhausted T cells, we hypothesized that T cells generated with αCD40 became exhausted in the TME and as such benefitted by reinvigoration with ICB. To test this hypothesis we tracked cytokine production of PD-1+ T cells (exhausted) vs. PD-1− T cells. Without therapy, both PD-1+ and PD-1− subsets were capable of some cytokine production (30.8 to 39.4 ± 9.1 to 12.2% SD for CD8+ T cells, 13.4 to 36.2 ± 8.5 to 15.6% SD for CD4+ T cells), with no significant differences between PD-1+ and PD-1− subsets (Fig. 4D and SI Appendix, Fig. S2 C and D). As with the total T cell population, treatment with either αCD40 or ICB did not significantly alter the proportion of cytokine producers between the two PD-1 subsets; however, the combination of αCD40/ICB significantly increased the proportions of cytokine producers in both PD-1 subsets (P = 0.04 for PD-1+ and P = 0.03 for PD-1− CD8+ T cells, P = 0.0008 for PD-1+ and P = 0.013 for PD-1− CD4+ T cells; Fig. 4D and SI Appendix, Fig. S2 C and D). These data raised the hypothesis that the PD-1+ subsets of CD4+ and CD8+ T cells are not functionally exhausted, even in control-treated mice, and that αCD40/ICB does not specifically reinvigorate the PD-1+ subset.
ICB Does Not Reinvigorate T Cells.
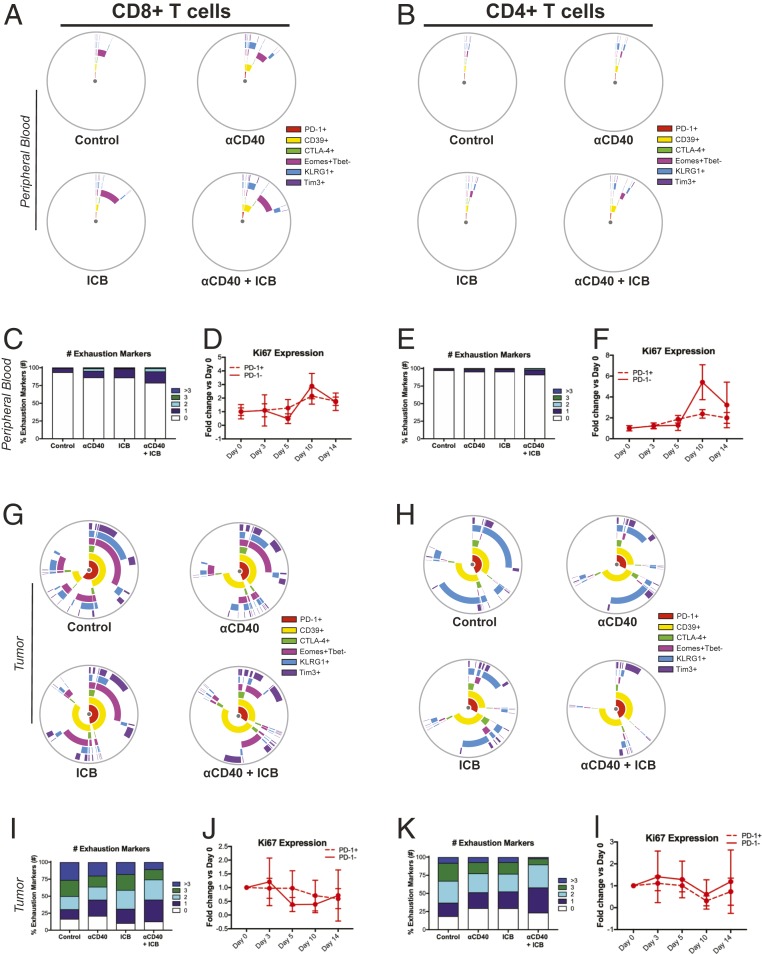
Given that PD-1+ T cells produced effector cytokines as efficiently as PD-1− T cells after combination αCD40/ICB therapy, we hypothesized that ICB contributed to therapeutic efficacy by enhancing the priming rather than via reinvigoration of the T cell response. To determine if T cell reinvigoration was a major determinant of ICB administration in the context of αCD40, we interrogated T cell subsets with regard to exhaustion marker expression in the peripheral blood 10 d after the start of therapy. We observed low expression of canonical exhaustion markers such as PD-1, Tim-3, and CTLA-4, with proportions of CD4+ and CD8+ T cells expressing these markers ranging from 0.03 to 1.9% for CD8+ T cells and 0.01 to 1.4% for CD4+ T cells, regardless of treatment (Fig. 5 A and B). The proportion of Lag3+ CD4+ or CD8+ T cells was less than 0.1%, also regardless of therapeutic intervention. The exhaustion-associated ectonuclease CD39 (32) was expressed at slightly higher proportions in control-treated mice (1.2 ± 0.7% SD in CD8+ T cells and 0.7 ± 0.7% SD in CD4+ T cells), with slight (but not significant) increases in the proportions of CD39+ cells upon αCD40/ICB treatment, regardless of ICB administration (9.3 ± 4.6% SD in CD8+ T cells, 5.3 ± 0.7% SD in CD4+ T cells; Fig. 5 A and B). Similarly, KLRG1 expression was low in control-treated mice (0.3 ± 0.1% SD in CD8+ T cells, 0.2 ± 0.1% SD in CD4+ T cells) and increased after αCD40/ICB administration (5.9 ± 2.8% SD in CD8+ T cells, 1.1 ± 0.7% SD in CD4+ T cells), but the changes were not statistically significant (Fig. 5 A and B). Indeed, only the Eomes+Tbet− subset of CD8+ T cells (susceptible to reinvigoration with αPD-1 blockade) (33) was significantly increased in the peripheral blood after αCD40/ICB (2.9 ± 0.7% SD in control-treated vs. 8.7 ± 1.6% SD in αCD40/ICB-treated mice, P = 0.0001) (Fig. 5 A and B). Furthermore, only a minority of CD4+ or CD8+ T cells expressed any combination of exhaustion markers, with the largest subset of exhausted cells expressing only a single marker (16.0% in the CD8+ T cells from αCD40/ICB-treated mice) (Fig. 5 C and E).
Fig. 5.
ICB does not reinvigorate T cells in the TME. Mice were implanted with s.c. PDA and treated as in SI Appendix, Fig. S1A. Peripheral blood was analyzed at day 10 after the start of therapy (A–C and E) or as indicated (D and F). Tumors were harvested and analyzed at day 14 after the start of therapy (G–I and K) or at indicated time points (J and L). (A and B) Mean percentage of live CD45+CD3+ CD8+ (A) or CD4+ (B) T cells in the peripheral blood that are positive for the indicated marker shown on each ring with each treatment group shown on an individual graph. Overlap of rings indicates coexpression of indicated markers. (C and E) Mean proportion of T cells expressing indicated number of exhaustion markers from A and B, respectively. (D and F) Time course indicating the fold change in the proportion of live CD45+CD3+Ki67+ CD8+ (D) or CD4+ (F) T cells in the peripheral blood at indicated time points, segregated by PD-1+ or PD-1− status as indicated. (G and H) Mean percentage of live CD45+CD3+ CD8+ (G) or CD4+ (H) T cells in the tumor that are positive for the indicated marker shown on each ring and each treatment group shown on individual graphs. Overlap of rings indicates coexpression of indicated markers. (I and K) Mean proportion of T cells expressing indicated number of exhaustion markers from G and H, respectively. (J and L) Time course indicating the fold change in the proportion of live CD45+CD3+Ki67+ CD8+ (J) or CD4+ (L) T cells in the peripheral blood at indicated time points, segregated by PD-1+ or PD-1− status as indicated. For timecourse analyses, treatment initiation was staggered such that mice were were killed on the same day. Ki67 graphs depict the fold change over the baseline expression of Ki67 at day 0 (the start of treatment). Each symbol represents the mean of indicated groups with the error bars indicating the SD (timecourse plots). All data are representative of n = 2 or 3 independent experiments with n = 5 to 8 mice per group.
To assess differences in the proliferative capacity of T cell subsets expressing exhaustion markers as a marker of reinvigoration, we interrogated the proportion of Ki67+ cells among the PD-1+ and PD-1− subsets at various time points after the start of therapy. PD-1+ T cells were no more or less proliferative as compared to PD-1− T cells in either the CD4+ or CD8+ T cell populations, either in control or treated mice (Fig. 5 D and F). Thus, the T cell population in the periphery of mice bearing 4662 tumors is minimally exhausted and not selectively reinvigorated after therapy.
We next investigated the exhaustion status of CD8+ and CD4+ T cells in the tumor site 14 d after the initiation of therapy. In contrast to T cells in the peripheral blood, we found that the majority of both CD8+ and CD4+ T cells expressed exhaustion markers in the TME, regardless of treatment (Fig. 5 G and H; Lag3 expression ranged from 0.1 to 3.9 ± 0.05 to 1.3% SD maximum among CD4+ and CD8+ T cell subsets). High proportions of CD4+ and CD8+ T cells expressed CD39, PD-1, and Tim3 in control-treated mice (CD39: 40.9 ± 8.7% SD in CD8+ T cells, 69.8 ± 2.4% SD in CD4+ T cells; PD-1: 46.8 ± 14.5% SD in CD8+ T cells, 31.9 ± 5.6% SD in CD4+ T cells; Tim3: 19.5 ± 9.3% SD in CD8+ T cells, 9.9 ± 2.9% SD in CD4+ T cells), but the addition of either ICB or αCD40 therapy did not significantly alter expression of these markers, even when combined together (CD39: 52.9 ± 19.0% SD in CD8+ T cells, 65.8 ± 10.5% SD in CD4+ T cells; PD-1: 19.0 ± 6.2% SD in CD8+ T cells, 34.0 ± 17.8% SD in CD4+ T cells; Tim3: 19.5 ± 9.3% SD in CD8+ T cells, 11.4 ± 3.3% SD in CD4+ T cells in αCD40/ICB-treated mice). A subset of Eomes+Tbet− CD8+ T cells was also highly represented in control-treated mice (51.1 ± 17.5% SD) but was also not significantly reduced with αCD40/ICB therapy (25.0 ± 8.0% SD; Fig. 5G). In contrast, a large subset of KLRG1+ cells observed in both the CD4+ and CD8+ T cell compartment of control-treated mice (32.4 ± 17.2% SD in CD8+ T cells, 53.1 ± 7.1% SD in CD4+ T cells) was significantly reduced after αCD40/ICB treatment (5.5 ± 2.0% SD in CD8+ T cells, 9.0 ± 7.3% SD in CD4+ T cells, P = 0.007 for CD8+ T cells and P < 0.0001 for CD4+ T cells comparing αCD40/ICB vs. control treatment), but not with either αCD40 or ICB alone.
Similarly, when analyzing the proportions of T cells expressing multiple exhaustion markers, we observed a slight trend but no significant reduction in the proportions of CD4+ or CD8+ T cells expressing three or more exhaustion markers (Fig. 5 I and K). Finally, again using Ki67 expression as a marker of exhausted T cell reinvigoration, we interrogated the proportion of PD-1+ or PD-1− T cells actively proliferating. We found that in both CD4+ and CD8+ T cells, the proportion of Ki67+ cells was similar, regardless of PD-1 status, after αCD40/ICB therapy (Fig. 5 J and L). Together, these data do not provide strong evidence for reinvigoration of exhausted T cells after treatment ICB even in combination with αCD40, suggesting that T cell priming is the critical effect of this approach.
Discussion
We have shown here that the addition of single-agent αCD40 to the combination of αPD-1 and αCTLA-4 can induce long-term, T cell-dependent cures in checkpoint-resistant PDA. This is important because current clinical trials with CD40 in pancreatic cancer utilize combinations with chemotherapy (NCT03214250), which is likely over time to degrade the memory response, as we have also shown here. Our data show that αCD40 potentiates ICB without the need for chemotherapy or radiation therapy and, importantly, does so without requiring innate immune sensing via TLR, STING, or IFNAR activation. As such, CD40 is molecularly set apart from both PRRs and immune checkpoint as a means of immune activation. Although αCD40 alone promoted systemic T cell activation and reduced immunosuppressive regulatory T cells in the TME, both αCD40 and ICB were required for increased proportions of functional effector CD4+ and CD8+ T cells. In this “cold” tumor model, we found little evidence for functionally exhausted T cells, and as such dual ICB functioned to enhance the quality of the effector T cell subsets rather than reverse exhaustion. Thus, CD40 activation represents a proximal immunostimulatory pathway that synergizes with ICB for T cell priming independently of PAMP or DAMP signaling.
Our data demonstrate nonredundant roles for CD40 agonism and ICB in priming a robust T cell response against PDA. Previous studies combining ICB and αCD40 in immunologically “hot” tumor models reveal a rationale for combining αPD-1/PD-L1 blockade with αCD40 as a result of IFN-γ–mediated up-regulation of PD-L1 expression in the TME (34) or IL-12–mediated down-regulation of PD-1 expression on T cells (and subsequent exhaustion reversal) in the TME (35, 36). Here, we show αCD40 alone was sufficient to increase T cell activation systemically with expected priming phase kinetics, consistent with previous reports by our group and others that αCD40 increases both the diversity and the clonality of the T cell response in the TME (22) and drives systemic activation of immune cells (37), indicative of a priming event. Both CD4+ and CD8+ T cells are required for maximal efficacy, consistent with CD4 T cell-mediated immune responses after αCD40 in breast cancer (37, 38), requiring further investigation in PDA. Despite evidence of a systemic priming event, the T cell response generated after agonist CD40 was suboptimal until ICB was added. High-quality effector T cells, as well as regression and cures of established tumors, were only observed in mice that received the synergistic combination αCD40/ICB.
Despite observations of anti-PD-1–induced reinvigoration of peripheral T cells (33, 39, 40), our data reveal a role for ICB-mediated augmentation of T cell function independently of reinvigoration. Previous reports cite roles for targeting ICB molecules in primary T cell responses: Loss of PD-1 or CTLA-4 signaling during the priming phase enhances the magnitude and quality of primary and memory T cell responses in both viral infections and murine tumor models (41–45), and we have reported αCTLA-4-mediated expansion of the anti-tumor T cell repertoire (46). A recent report reveals PD-1 blockade recruits new T cell receptor clonotypes to the TME in basal and squamous cell carcinomas, indicative of a new “wave” of immune editing in an immunologically hot tumor (47), and previous studies report enhanced T cell responses when combining TLR stimulation with ICB during the priming phase (48, 49). Although we cannot exclude some potential for ICB-mediated reinvigoration of T cell populations in PDA, we do not observe significantly altered exhaustion marker profiles or proliferative dysfunction within the PD-1+ subset after ICB. Additional longitudinal studies are needed to assess the activation and proliferation phenotype of T cells in the peripheral blood as a biomarker of response to αCD40/ICB therapy in PDA. Recent reports highlighting the distinct cellular mechanisms observed only in certain T cell populations after dual ICB as compared to either αPD-1 or αCTLA-4 alone bolster our finding that both ICB molecules were required in combination with αCD40 for optimal survival benefit in the orthotopic tumor setting (50, 51). Together, these data support a model whereby priming of both CD4+ and CD8+ T cell subsets (via αCD40) and the removal of negative feedback signals on the newly primed T cells [via PD-1/PD-L1 or CTLA-4/CD80 blockade, or alleviation of CD28 inhibition (52)] generated potent and functional T cell responses in immunologically “cold” PDA.
We have previously reported that chemotherapy or radiation therapy combined with αCD40 in PDA drives T cell responses in combination with ICB without the need for innate immune sensors, B cells, or type I IFN signaling (22, 23, 25). However, we show here that neither chemotherapy nor radiation therapy is necessary. As noted, chemotherapy is potentially even detrimental to therapeutic efficacy when αCD40 is combined with ICB, likely as a result of chemotherapy-mediated preferential depletion of naïve and proliferating T cells and negative long-term impact on the induction of long-term memory responses (53, 54). The liberation of DAMPs and PAMPs as a result of chemotherapy can sensitize tumors to ICB via TLR-dependent DC activation (55, 56), and more aggressive tumor models benefit from combining ICB with TLR or STING agonists to increase ICB sensitivity in preclinical studies (48, 57, 58). We show here that αCD40/ICB was sufficient to drive T cell responses in the absence of innate immune sensor stimuli, independently of TLR and STING signaling pathways. These data highlight the potency of αCD40 as a T cell priming agent and, given the orthogonality, suggest therapeutic combinations could be further enhanced by using αCD40/ICB with STING agonists (48, 57, 58), or in cell- or neoepitope-based vaccines (38). Indeed, the greatest opportunity for therapeutic success in PDA may be through combinatorial approaches, as a number of immunosuppressive mediators in the KPC TME—including CXCL1 (26) and COX2 (59)—subvert the efficacy of αCD40/ICB when administered in the absence of chemotherapy or radiation therapy.
Amid the burgeoning growth of positive clinical trials and approvals for ICB, there remains a large number of patients with tumors who are unresponsive to ICB and a tremendous unmet need for effective combinations. From data reported here and elsewhere, we hypothesize that αCD40, when used in combination with dual ICB, is an alternative bridge between innate and adaptive immunity and may extend the reach of cancer immunotherapy without requiring chemotherapy or radiation therapy to release DAMP-activating molecules. With the advent of clinical-grade αCD40 investigational agents, this hypothesis can be directly tested in patients. Early results are promising (12, 60–62).
Methods
Mice.
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania; 6- to 8-wk-old female and male C57BL/6, Batf3 KO (B6.129S(C)-Batf3tm1Kmm/J) (27), CD40 KO (B6.129P2-Cd40tm1Kik/J) (63), MyD88 KO (B6.129P2(SJL)-Myd88tm1.1Defr/J) (64), STING KO (B6(Cg)-Tmem173tm1.2Camb/J) (65), and IFNAR KO (B6.129S2-Ifnartm1Agt/Mmjax) (66) mice used for implantable tumor studies were purchased from The Jackson Laboratory.
Cell Lines and In Vivo Tumor Growth.
The mouse PDA cell line 4662 was derived from a KrasLSL-G12D/+,Trp53LSL-R172H/+,Pdx1-Cre (KPC) mouse (67) as previously described (68, 69) and used in experiments after six passages in vitro. The colony of KPC was tested by high-resolution single-nucleotide polymorphism testing and found to be congenic to C57BL/6 (22). C57BL/6 mice received s.c. injections of 4662 only if tumor cell viability was >90%. B16-F10 was purchased from ATCC and passaged four times in vitro. Cell lines were tested by using the Infectious Microbe PCR Amplification Test and authenticated by the Research Animal Diagnostic Laboratory at the University of Missouri. Tumors were measured thrice weekly by calipers, and the volume was calculated as (L × W2)/2, where L is the longest diameter and W is the perpendicular diameter. Endpoint criteria for the survival studies included tumor volume exceeding 500 mm3. Mice that died suddenly or developed large tumor ulceration were censored on the day of death or killing.
For implantable tumor experiments, PDA tumor cells (2.5 × 105) and B16-F10 cells (5 × 104) were resuspended in Dulbecco’s modified Eagle’s medium (DMEM), injected s.c. into the flanks of mice, and allowed to grow 14 to 21 d until tumor volumes averaged 30 to 60 mm3. Mice were then assigned to treatment groups by block randomizing by cage such that cohorts were balanced for baseline tumor size.
For orthotopic experiments, PDA tumor cells (1.25 × 105) were injected in DMEM s.c. into the pancreata of mice and allowed to grow 14 d, at which point therapy was initiated. Tumors were visualized weekly by ultrasonography (Vevo 2100 Imaging System with 55-MHz MicroScan transducer; Visual Sonics) and tumor volume was quantified using the integrated Vevo Workstation software package. Endpoint criteria included tumor volume exceeding 1,000 mm3 (by ultrasonography), severe cachexia, or extreme weakness and inactivity.
Murine In Vivo Antibodies.
Mice were treated intraperitoneally (i.p.) with αPD-1 (RMP1-14, 200 μg per dose; BioXcell) on days 0, 3, 6, 9, 12, and 15 (after enrollment) and αCTLA-4 (9H10, 200 μg per dose; BioXcell) on days 0, 3, and 6. Agonistic αCD40 (FGK45, 100 μg; BioXcell) was given on day 3. For T cell depletion studies, αCD8 (2.43, 200 μg per dose; BioXcell) and αCD4 mAbs (GK1.5, 200 μg per dose; BioXcell) were injected twice weekly for the duration of the experiment, starting on day −1 (day of enrollment). For isotype controls, rat IgG2a (2A3; BioXcell; 200 μg) and rat IgG2b (LTF-2; BioXcell; 200 μg per dose) were used. This approach achieved >98% depletion of CD8+ and CD4+ T cells in peripheral blood and tumor tissue compared with that of control mice, as monitored by flow cytometry. For tumor rechallenge studies, αCD8, αCD4, or isotype control antibodies were injected i.p. the day before the rechallenge and continued twice weekly until day 60 or the mouse was killed for tumor burden. All antibodies were endotoxin-free.
Collection of Tissue Samples from Mice.
Mice were killed and tissues were harvested 5, 10, or 14 d after onset of therapy (as indicated in figure legends). The entire s.c. tumor was washed in PBS, minced into small fragments, and incubated in collagenase XI with protease inhibitor (1 mg/mL in DMEM; Sigma-Aldrich) at 37 °C for 45 min with rotary agitation. Dissociated cells were passed through a 70-μm strainer and washed three times in phosphate-buffered saline (PBS) with 0.5% bovine serum albumin (BSA) and 2 mM ethylenediaminetetraacetic acid (EDTA). Spleens and lymph nodes were homogenized and passed through a 70-μm cell strainer to achieve single-cell suspensions. For spleens, red blood cells were lysed using ACK Lysis Buffer (BioWhittaker). Cells were counted using the Beckman Coulter Counter Z2.
Flow Cytometry.
Cell-surface molecules were analyzed by incubating single-cell suspensions of tissues with Live/Dead Fixable Aqua (Thermo Fisher Scientific) for 10 min at room temperature followed by incubation with primary fluorochrome-labeled antibodies at 4 °C for 30 min in PBS with 0.5% BSA and 2 mM EDTA. For cytokine production by T cell subsets, samples were incubated for 4 h at 37 °C with PMA (20 ng/mL; Sigma), ionomycin (1 μg/mL; Sigma), and Brefeldin A (BD Biosciences). Intracellular staining was done using the Fixation/Permeabilization Kit (Thermo Fisher Scientific). Antibodies used in flow analysis are listed in SI Appendix, Table S1. Flow cytometric analysis was performed on a FACSCanto or LSR Fortessa Flow Cytometer (BD Biosciences). Collected data were analyzed using FlowJo v10 (TreeStar).
Statistical Analysis.
Differences between two groups were analyzed by a two-tailed Student’s t test. Differences between three or more groups were analyzed by one-way ANOVA with the Tukey post hoc test to correct for multiple comparisons to assess differences between any two groups. Tumor growth curves were analyzed by two-way ANOVA, with Tukey multiple comparisons of means used as a post hoc test to assess differences between any two groups. Survival curves were assessed by the log rank (Mantel–Cox). All statistical analyses were performed on GraphPad Prism 6 (GraphPad) or R version 3.4. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 unless otherwise indicated).
Data Availability Statement.
All data are contained in the paper or SI Appendix.
Supplementary Material
Acknowledgments
We thank members of the R.H.V. and Wherry laboratories, especially Dr. Alexander Huang for helpful scientific discussions, as well as Dr. Cynthia Clendenin for additional support. This work was supported by the Parker Institute for Cancer Immunotherapy (K.T.B. and R.H.V.) and National Institutes of Health grants TL1TR001880 (A.H.M.), K12 CA076931 (M.S.D.), K08 CA241084 (M.S.D.), R01CA229803 (R.H.V.), and P01CA210944 (R.H.V.).
Footnotes
Competing interest statement: R.H.V. has received consulting fees or honoraria from Apexigen, AstraZeneca, Celgene, Genentech, Janssen, Lilly, Medimmune, Merck, and Verastem; he has received research funding from Apexigen, Fibrogen, Inovio, Janssen, and Lilly. He is an inventor on a licensed patent relating to cancer cellular immunotherapy and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918971117/-/DCSupplemental.
References
- 1.Janeway C. A. Jr, Medzhitov R., Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Janeway C. A., Jr, Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Geisse J. K., et al. , Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study. J. Am. Acad. Dermatol. 47, 390–398 (2002). [DOI] [PubMed] [Google Scholar]
- 4.van Seters M., et al. , Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N. Engl. J. Med. 358, 1465–1473 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Ott P. A., et al. , An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A., Wolchok J. D., Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber J. S., et al. , Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer 115, 3944–3954 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A., et al. , Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: A phase II study. Neuro Oncol. 12, 401–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsh V., et al. , Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2667–2674 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G., Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Woo S. R., Corrales L., Gajewski T. F., Innate immune recognition of cancer. Annu. Rev. Immunol. 33, 445–474 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide R. H., CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Vonderheide R. H., Glennie M. J., Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19, 1035–1043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotomayor E. M., et al. , Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 5, 780–787 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Diehl L., et al. , CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 5, 774–779 (1999). [DOI] [PubMed] [Google Scholar]
- 16.French R. R., Chan H. T., Tutt A. L., Glennie M. J., CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5, 548–553 (1999). [DOI] [PubMed] [Google Scholar]
- 17.van Mierlo G. J., et al. , CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl. Acad. Sci. U.S.A. 99, 5561–5566 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho H. I., Celis E., Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 69, 9012–9019 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahonen C. L., et al. , Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood 111, 3116–3125 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak A. K., Robinson B. W., Lake R. A., Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 63, 4490–4496 (2003). [PubMed] [Google Scholar]
- 21.Honeychurch J., Glennie M. J., Johnson P. W., Illidge T. M., Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell-dependent immunity to B-cell lymphoma. Blood 102, 1449–1457 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Byrne K. T., Vonderheide R. H., CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 15, 2719–2732 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rech A. J., et al. , Radiotherapy and CD40 activation separately augment immunity to checkpoint blockade in cancer. Cancer Res. 78, 4282–4291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter E. L., Mick R., Rüter J., Vonderheide R. H., Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J. Transl. Med. 7, 93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winograd R., et al. , Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., et al. , Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49, 178–193.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildner K., et al. , Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupont C. D., et al. , Flt3 ligand is essential for survival and protective immune responses during toxoplasmosis. J. Immunol. 195, 4369–4377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harms Pritchard G., et al. , Diverse roles for T-bet in the effector responses required for resistance to infection. J. Immunol. 194, 1131–1140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupont C. D., et al. , Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS Pathog. 10, e1004047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson T. R., et al. , Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canale F. P., et al. , CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 78, 115–128 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Huang A. C., et al. , T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zippelius A., Schreiner J., Herzig P., Müller P., Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol. Res. 3, 236–244 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Garris C. S., et al. , Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49, 1148–1161.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngiow S. F., et al. , Agonistic CD40 mAb-driven IL12 reverses resistance to anti-PD1 in a T-cell-rich tumor. Cancer Res. 76, 6266–6277 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Spitzer M. H., et al. , Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma H. S., et al. , A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol. Res. 7, 428–442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamphorst A. O., et al. , Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. U.S.A. 114, 4993–4998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieg C., et al. , High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 24, 144–153 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Allie S. R., Zhang W., Fuse S., Usherwood E. J., Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J. Immunol. 186, 6280–6286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blank C., et al. , PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 64, 1140–1145 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Peggs K. S., Quezada S. A., Chambers C. A., Korman A. J., Allison J. P., Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers C. A., Kuhns M. S., Allison J. P., Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4(+) T cell responses. Proc. Natl. Acad. Sci. U.S.A. 96, 8603–8608 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrikant P., Khoruts A., Mescher M. F., CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 11, 483–493 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Twyman-Saint Victor C., et al. , Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yost K. E., et al. , Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahm C. D., Colluru V. T., McIlwain S. J., Ong I. M., McNeel D. G., TLR stimulation during T-cell activation lowers PD-1 expression on CD8+ T cells. Cancer Immunol. Res. 6, 1364–1374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahm C. D., Colluru V. T., McNeel D. G., Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol. Res. 5, 630–641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gide T. N., et al. , Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Wei S. C., et al. , Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. U.S.A. 116, 22699–22709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui E., et al. , T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackall C. L., T-cell immunodeficiency following cytotoxic antineoplastic therapy: A review. Oncologist 4, 370–378 (1999). [PubMed] [Google Scholar]
- 54.McCoy M. J., Lake R. A., van der Most R. G., Dick I. M., Nowak A. K., Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br. J. Cancer 107, 1107–1115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfirschke C., et al. , Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44, 343–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roses R. E., Datta J., Czerniecki B. J., Radiation as immunomodulator: Implications for dendritic cell-based immunotherapy. Radiat. Res. 182, 211–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin P., et al. , CpG-induced antitumor immunity requires IL-12 in expansion of effector cells and down-regulation of PD-1. Oncotarget 7, 70223–70231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ager C. R., et al. , Intratumoral STING activation with T-cell checkpoint modulation generates systemic antitumor immunity. Cancer Immunol. Res. 5, 676–684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markosyan N., et al. , Tumor cell-intrinsic EPHA2 suppresses anti-tumor immunity by regulating PTGS2 (COX-2). J. Clin. Invest. 130, 3594–3609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bajor D. L., et al. , Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. OncoImmunology 7, e1468956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Hara M. H., et al. , Abstract CT004: A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine and nab-paclitaxel with or without nivolumab in untreated metastatic ductal pancreatic adenocarcinoma patients. Cancer Res., 10.1158/1538-7445.AM2019-CT004 (2019). [DOI] [Google Scholar]
- 62.Beatty G. L., et al. , CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawabe T., et al. , The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Hou B., Reizis B., DeFranco A. L., Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin L., et al. , MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 187, 2595–2601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller U., et al. , Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994). [DOI] [PubMed] [Google Scholar]
- 67.Hingorani S. R., et al. , Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Lo A., et al. , Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 75, 2800–2810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans R. A., et al. , Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight 1, 1–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained in the paper or SI Appendix.