Figure 2.
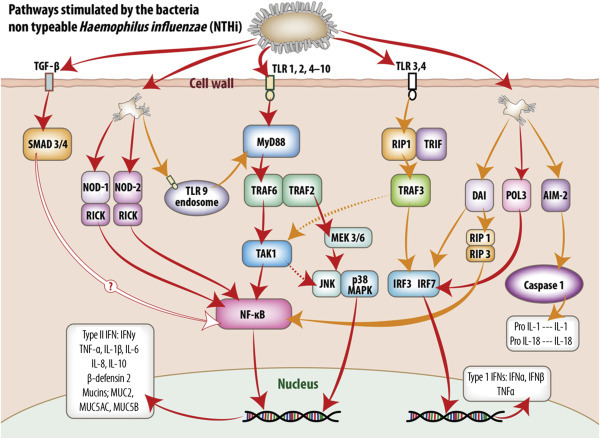
Intracellular signaling pathways utilized by middle ear mucosa in response to stimulation by non-typeable Haemophilus influenzae (NTHi).
Pattern recognition receptors (PRR) detect conserved molecular signatures of invading microbes and facilitate activation of synthesis and secretion of downstream cytokine cascades. This figure schematically represents the signaling pathways activated by NTHi both at the cell membrane and within the cell cytoplasm of the middle ear mucosal cells. The information portrayed was extracted from published reports of experiments utilizing middle ear cell lines and middle ear epithelium and mucosa from animal models and human biopsy material. Transmembrane TLR receptors 1, 2, and 4–10 predominate at the cell surface and their activation results in translocation of NF-κB to the nucleus and upregulation of type II interferons and a range of proinflammatory and antimicrobial molecules. TLR3 utilizes an MYD88-independent pathway via TRIF and IRF3 to upregulate type I interferons. In addition, stimulation of TLR3 and 4 may also activate a delayed response (dotted lines) through TAK1, then the JNK p38MAPK pathway or NF-κB to stimulate type II interferons and proinflammatory molecule production. TGF-β activates SMAD 3 and SMAD 4, ultimately upregulating NF-κB; however, the mechanism for this pathway, although assumed similar to that exhibited by respiratory epithelia, has not been confirmed for middle ear mucosa as indicated. PRR within the cell cytoplasm, such as NOD-1 and NOD-2 and TLR9 receptors on the endosomes, can also upregulate NF-κB stimulation of type II interferons and proinflammatory and antimicrobial molecules via MyD88-independent and -dependent pathways, respectively. DAI may activate both NF-κB and NF-κB-independent stimulation of type II and type 1 interferons, respectively. POL3 via IRF7 stimulates the MyD88-independent pathway utilized by TLR3, while AIM2, via caspase 1, stimulates formation of IL1 and IL18 formation within the cytoplasm.
