Abstract
Dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN). DC-SIGN is a C-type lectin receptor that recognizes N-linked high-mannose oligosaccharides and branched fucosylated structures. It is now clear that the biological role of DC-SIGN is two-fold. It is primarily expressed by dendritic cells and mediates important functions necessary for the induction of successful immune responses that are essential for the clearance of microbial infections, such as the capture, destruction, and presentation of microbial pathogens to induce successful immune responses. Yet, on the other hand, pathogens may also exploit DC-SIGN to modulate DC functioning thereby skewing the immune response and promoting their own survival. This chapter presents an overview of the structure of DC-SIGN and its expression pattern among immune cells. The current state of knowledge of DC-SIGN-carbohydrate interactions is discussed and how these interactions influence dendritic cell functioning is examined. The molecular aspects that underlie the selectivity of DC-SIGN for mannose-and fucose-containing carbohydrates are detailed. Furthermore, the chapter discusses the role of DC-SIGN in dendritic cell biology and how certain bacterial pathogens exploit DC-SIGN to escape immune surveillance.
Summary
Dendritic cell-specific ICAM-3-grabbing non-integrin (DC-SIGN) is a C-type lectin receptor that recognizes N-linked high-mannose oligosaccharides and branched fucosylated structures. It is primarily expressed by dendritic cells and mediates the capture, destruction and presentation of microbial pathogens to induce successful immune responses. Furthermore, DC-SIGN is involved in the priming of T-cell responses and facilitates dendritic cell homeostasis by controlling extravasation into peripheral tissues. However, an increasing amount of evidence suggests that pathogens also exploit DC-SIGN to subvert host immune responses. Herein, we discuss the current state of knowledge of DC-SIGN-carbohydrate interactions and investigate how these interactions influence dendritic cell functioning. First, an overview of the structure of DC-SIGN is provided and its expression pattern among immune cells is discussed. After this, the molecular aspects that underlie the selectivity of DC-SIGN for mannose-and fucose-containing carbohydrates are detailed. Finally, the role of DC-SIGN in dendritic cell biology is discussed and how certain bacterial pathogens exploit DC-SIGN to escape immune surveillance.
Keywords: C-Type lectin; Dendritic cell-specific ICAM-3-grabbing non-integrin (DC-SIGN); High-mannose; Lewis antigens; Carbohydrate recognition; Dendritic cell; Immune suppression; Host-pathogen interaction; Pathogenesis; Virulence
1. Introduction
The capability of the innate immune system quickly to detect and respond to invading pathogens is essential for controlling infection. Pattern-recognition receptors (PRRs) have evolved to recognize a wide variety of pathogen-associated molecular patterns (PAMPs) and trigger antimicrobial responses in immune cells. Recognition of PAMPs by PRRs is an important determinant for the overall quality and effectiveness of immune responses by mediating not only direct effector functions, such as phagocytosis and degranulation, but also by transmitting signals that regulate the expression of genes important for both innate and adaptive immune responses. One of the best studied classes of PRRs are the Toll-like receptors (TLRs). This receptor family recognizes a diverse range of microbial compounds and, upon ligation, trigger robust innate immune responses by inducing proinflammatory cytokine and chemokine production and by upregulating co-stimulatory molecule expression (Akira and Takeda, 2004). Although TLRs are an important warning system that alerts host immune cells to the presence of invading microbes, it remains unclear whether they also facilitate antigen uptake and it seems that this process is mediated by other types of PRRs, such as scavenger receptors (Murphy et al., 2005) and C-type lectin receptors (CLRs) (Cambi et al., 2005; McGreal et al.,2005). The C-type lectin receptors encompass a large family of proteins that are best known for their ability to detect and capture microbe-derived materials. Classical CLRs contain calcium- (Ca2+-) dependent carbohydrate recognition domains (CRDs) which are involved in the recognition of carbohydrate structures on both self and non-self ligands. The C-type lectin CRDs are part of a large family of protein domains called C-type lectin domains (CTLDs) which are characterized by a common protein-fold consisting of two anti-parallel β-strands and two α-helices (Weis et al., 1998; Drickamer, 1999). Although the name C-type lectin refers to a Ca2+-dependent carbohydrate-binding protein, it is now clear that this name is a misnomer and that many C-type lectins are calcium-independent and do not bind carbohydrates but rather recognize proteins or lipids. Therefore, the term C-type lectin does not refer to a protein family which shares functional similarities but rather defines a class of proteins that all contain one or more CTLDs.
The C-type lectin receptors exhibit important functions during primary immune responses (Robinson et al., 2006). Besides their role in pathogen detection and uptake via PAMPs, they also mediate cell–cell interactions by the recognition of endogenous ligands (Cambi and Figdor, 2003). Furthermore, they are involved in the induction of immune tolerance and play a role in endogenous glycoprotein homeostasis (Cambi and Figdor, 2003). In addition, C-type lectins have been shown to influence TLR-mediated responses (Mukhopadhyay et al., 2004; Cambi et al., 2005). Moreover, C-type lectins can be produced as transmembrane proteins or as soluble, secreted proteins. Examples of soluble C-type lectins are the members of the collectins family, such as lung surfactant proteins A and D (Pastva et al., 2007), and the plasma-localized mannose-binding lectin (MBL) (Takahashi et al., 2006) (see Chapter 35). Transmembrane C-type lectins can be divided into two groups based upon the orientation of their amino (N)-terminus. Myeloid cells predominantly express type-II C-type lectins. These lectins all contain a single CRD and have been shown to bind a wide variety of carbohydrate ligands. Examples of myeloid type-II C-type lectins are the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and its close relative DC-SIGNR (L-SIGN), the related murine receptor family termed SIGN-related (SIGNR) 1-4, Langerin, blood DC antigen 2 (BDCA-2), C-type lectin receptors 1 and 2 (CLEC-1 and CLEC-2, respectively), macrophage galactose-type lectin (MGL), DC immunoreceptor (DCIR) and Dectin-1 and -2 (Cambi et al., 2005; McGreal et al., 2005; Robinson et al., 2006). Myeloid type-I C-type lectins contain multiple CRDs. Examples of these lectins are the macrophage mannose receptor (MR), the phosholipase A2 receptor, DEC-205 and Endo-180 (Apostolopoulos and McKenzie, 2001; East and Isacke, 2002). Investigations on the carbohydrate-binding specificities of Ca2+-dependent CRDs have shown that they fall within two broad categories: those recognizing d-mannose- (Man-) like structures and those recognizing d-galactose- (Gal-) like structures. This subdivision is supported at the molecular level by studies demonstrating that Man- and Gal-binding CRDs contain characteristic amino acid triplets: EPN (in one-letter amino-acid code) for Man-binding CRDs and QDP (in one-letter amino-acid code) for Gal-binding CRDs (Drickamer, 1992). In addition, some CLRs (e.g. MR) can recognize sulfated carbohydrates independent of their CRD. This interaction is mediated by a cysteine-rich domain which is present in these proteins (Fiete et al., 1998).
Indeed, DC-SIGN is a dendritic cell (DC)-specific type-II C-type lectin which was originally identified as a receptor that specifically interacts with the intercellular adhesion molecule (ICAM)-3 on T-cells, thereby mediating DC–T-cell interactions (Geijtenbeek et al., 2000b). Later on, DC-SIGN was also shown to be involved in DC migration by interacting with ICAM-2 on vascular endothelial cells (Geijtenbeek et al., 2000a). Ever since its discovery, DC-SIGN received major scientific interest. This interest grew further when it became clear that DC-SIGN has a dual function in that it also recognizes a wide variety of pathogens (Table 34.1 ). Investigations on its carbohydrate-specificity using glyco-arrays have shown that DC-SIGN preferentially binds Man- and l-fucose- (Fuc-) containing structures. In this chapter, we summarize the current state of knowledge on DC-SIGN-carbohydrate interactions. First, the structure of DC-SIGN and its expression patterns among various immune cells is discussed. Then the molecular aspects that underlie the selectivity of DC-SIGN for Man- and Fuc-containing carbohydrates are detailed. Finally, the role of DC-SIGN in DC biology and how certain pathogens exploit DC-SIGN to escape immune surveillance are reviewed.
Table 34.1.
Pathogens recognized by DC-SIGN
| Pathogen | Ligandb | Carbohydrate epitope |
|---|---|---|
| Virusesa | ||
| HIV-1 | gp120 | High-mannose |
| HIV-2 | gp120 | Unknown |
| SIV | gp120 | Unknown |
| FIV | gp95 | Unknown |
| Ebola virus | Glycoprotein | High-mannose |
| Marburg virus | Glycoprotein | Unknown |
| Cytomegalovirus | Glycoprotein B | Unknown |
| Hepatitis C virus | E1/E2 glycoproteins | Unknown |
| Dengue virus | Glycoprotein E | N-linked glycans |
| Alpha viruses | Unknown | Unknown |
| SARS corona virus | S protein | N-linked glycans |
| West Nile virus | Glycoprotein E | N-linked glycans |
| Human herpes virus 8 | Unknown | Unknown |
| Measles virus | Glycoproteins F and H | Unknown |
| Bacteria | ||
| Helicobacter pylori | LPS | Lewis antigens |
| Mycobacterium spp. | ManLAM, PIMs, LM, AM, 19- and 45-kDa antigens | Di- and tri-mannose |
| Lactobacillus spp. | S-layer protein A | Unknown |
| Escherichia coli | LPS | N-acetyl-d-glucosamine |
| Salmonella enterica | LPS | N-acetyl-d-glucosamine |
| Haemophilus ducreyi | LPS | N-acetyl-d-glucosamine |
| Neisseria spp. | lgtB LPS | N-acetyl-d-glucosamine |
| Streptococcus pneumoniae | Capsular polysaccharides and unknown | Unknown |
| Yersinia pestis | LPS | Unknown |
| Fungi | ||
| Candida albicans | N-linked mannan | Mannose |
| Aspergillus fumigatus | Unknown | Unknown |
| Chrysosporium tropicum | Unknown | Unknown |
| Parasites | ||
| Leishmania spp. | Unknown | Unknown |
| Schistosoma mansoni | SEAs and glycolipids | Lewisx and peudo-Lewisy |
| Toxocara canis | Excretory/secretory products | Unknown |
Viral abbreviations: HIV, human immunodeficiency virus; SIV, simian immunodeficiency virus; FIV, feline immunodeficiency virus; SARS, severe acute respiratory syndrome.
Compound abbreviations: AM, arabinomannan; LM, lipomannan; LPS, lipopolysaccharide; ManLAM, mannose-capped lipoarabinomannan; PIMs, phosphatidylinositol-mannosides; SEAs, soluble egg antigens.
2. DC-SIGN Structure and Expression
2.1. Structure of DC-SIGN
Human DC-SIGN (CD209) is a 404 amino acid protein encoded in seven exons on chromosome 19p13. Upstream, in the reverse orientation, and downstream of DC-SIGN, two DC-SIGN homologues are encoded: DC-SIGNR which shows 77% amino acid sequence identity to DC-SIGN and LSECtin which has 31% identity to DC-SIGN, respectively (Soilleux et al., 2000; Liu et al., 2004b). Further downstream lies the gene encoding the low affinity IgE receptor (CD23), a C-type lectin with a gene organization similar to DC-SIGN (Soilleux et al., 2000). Structurally, DC-SIGN itself is a prototype type II transmembrane protein consisting of a carboxy (C)-terminal CRD followed by a neck domain fused to a transmembrane region and ending with a N-terminal cytoplasmic tail, which harbours internalization and recycling motifs, such as a di-leucine (LL) and a tri-acidic (EEE) motif, and an incomplete immunoreceptor tyrosine-based activation motif (ITAM) (van Kooyk and Geijtenbeek, 2003) (Figure 34.1A ). Crystal structures of the CRDs of DC-SIGN and DC-SIGNR revealed that both CRDs exhibit a typical long-form C-type lectin fold (Feinberg et al., 2001) (Figure 34.1B). The structure harbours three Ca2+ ions, of which one, the principal Ca2+ (see Figure 34.1B; Ca2+ no. 2), is common to all Ca2+-dependent C-type lectins and dictates the recognition of specific carbohydrate structures. The principal Ca2+ interacts with four amino acids; Glu347, Asn349, Glu354 and Asn365 in DC-SIGN (where Glu, glutamic acid; Asn, asparagine) and Glu359, Asn361, Glu366 and Asn377 in DC-SIGNR and mutations in these amino acids lead to loss of ligand interaction (Geijtenbeek et al., 2002b). The DC-SIGN neck domain is composed of one incomplete and seven complete 23-amino acid tandem repeats which are encoded in a single exon. The number of neck repeats is highly conserved, although polymorphisms have been reported (Liu et al., 2004a; Barreiro et al., 2007; Ben-Ali et al., 2007; Rathore et al., 2008). In contrast, the number of neck repeats in DC-SIGNR is much more variable and varies between four and nine (Bashirova et al., 2001; Feinberg et al., 2005). Cross-linking and analytical ultracentrifugation experiments have shown that purified recombinant DC-SIGN and DC-SIGNR CRDs exist as monomers, whereas the complete extracellular domains form tetramers (Mitchell et al., 2001; Feinberg et al., 2005). Analysis by circular dichroism spectroscopy indicated that the neck region has a high α-helical content and forms a tetrameric coiled-coil structure that projects the CRDs away from the cell surface (Mitchell et al., 2001). Furthermore, it was demonstrated that tetramerization enhances the affinity for multivalent ligands (Mitchell et al., 2001). A disadvantage of using recombinant proteins is that they may exhibit differential glycosylation and therefore behave differently from their natural equivalents. In a recent study, which investigated the multimerization and ligand-binding activities of various DC-SIGN splice forms, it was shown that N-glycosylation of Asn80 in repeat 1 negatively influences multimer formation (Serrano-Gomez et al., 2008). Furthermore, it was demonstrated that the presence of the two most N-terminal repeats is sufficient for multimerization, that the specific order of the neck repeats is important for functionality and that the CRD contributes to tetramer stabilization via cysteine-mediated interactions (Serrano-Gomez et al., 2008). Interestingly, the authors also found that, although the capacity to multimerize correlates with the ability to bind soluble carbohydrates, it does not correlate with the capability to interact with certain pathogens, such as Candida albicans and Leishmania infantum (Serrano-Gomez et al., 2008). A possible explanation is that the large amount of DC-SIGN ligands on the surface of interacting pathogens compensates for the distinct affinities and multimerization abilities of DC-SIGN isoforms.
Figure 34.1.

Structure of DC-SIGN. (A) Schematic representation of DC-SIGN. DC-SIGN is a prototype type II transmembrane protein consisting of a carboxy- (COOH-) terminal carbohydrate recognition domain (CRD), followed by a neck domain consisting of one incomplete and seven complete tandem repeats (numbered 1–7.5) fused to a transmembrane domain (TMD) and ending with an amino- (NH3+-) terminal cytoplasmic tail. The latter harbours internalization and recycling motifs, such as a di-leucine (LL) and a tri-acidic (EEE) motif, and an incomplete immunoreceptor tyrosine- (Y-) based activation motif (ITAM). An asterisk indicates the N-glycosylation site situated in neck repeat 1. (B) Ribbon diagram of the CRD of DC-SIGN. Large spheres represent the three Ca2+ ions (ions are numbered Ca1–Ca3). Disulfide bonds are indicated by an asterisk. The CRD of DC-SIGNR resembles that of DC-SIGN, except for the disulfide bond connecting the carboxy- and amino-termini, which cannot be seen in DC-SIGNR.
Figure 34.1B was adapted, with permission, from Feinberg et al. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. © AAAS.
2.2. Expression of DC-SIGN
DC-SIGN is expressed by DCs both in vitroand in vivo (Bleijs et al., 2001). Monocyte- and CD34+-derived DCs abundantly express DC-SIGN (Geijtenbeek et al., 2000b). It is not expressed by monocytes, monocytic cell lines, activated monocytes, granulocytes, CD34+ bone marrow cells or T- and B-lymphocytes (Geijtenbeek et al., 2000b). In vivo, DC-SIGN is expressed by immature DCs (iDCs) in lymphoid and fibrous connective tissues and in mucosae, but not by epidermal CD1a+ Langerhans cells (Geijtenbeek et al., 2000a, Geijtenbeek et al., 2000c; Soilleux and Coleman, 2001; Soilleux et al., 2002). The percentage of DC-SIGN-positive blood cells is very low and only certain subsets of blood DCs seem to express DC-SIGN (Engering et al., 2002b; Soilleux et al., 2002). Although DC-SIGN was thought to be DC-specific, its expression has now been demonstrated on specific macrophage subsets, such as decidual and alveolar macrophages and on Hofbauer cells in the placenta (Geijtenbeek et al., 2001; Lee et al., 2001; Soilleux et al., 2001, Soilleux et al., 2002). Interestingly, studies in fetal tissues have demonstrated that while fetal iDCs normally express DC-SIGN, alveolar macrophages do not (Soilleux et al., 2002). This indicates that DC-SIGN expression in this type of macrophage may be dependent on antigenic stimulation. Consistent with this hypothesis is the finding that interleukin- (IL-) 13 treatment of monocyte-derived macrophages induces DC-SIGN expression (Soilleux et al., 2002). This observation, together with the ability of IL-4 to induce monocytic DC-SIGN expression (Relloso et al., 2002) and the genetic linkage between DC-SIGN and CD23, which is part of the T-helper-2 cell (Th2) axis of immunity, suggests that DC-SIGN plays a role in Th2-type immune responses (Soilleux et al., 2000; Park et al., 2001; Soilleux, 2003).
3. Selective Recognition of Man- and Fuc-Containing Glycans by DC-SIGN
Glycan array screening using recombinant DC-SIGN fragments or DC-SIGN extracellular domains fused to an Fc domain (DC-SIGN–Fc) have demonstrated that DC-SIGN recognizes two classes of carbohydrates: N-linked high-Man oligosaccharides and branched fucosylated structures such as the Lewis (Le) blood group antigens, Lea, Leb, Lex, and Ley (Feinberg et al., 2001, Feinberg et al., 2007; Appelmelk et al., 2003; Guo et al., 2004; van Liempt et al., 2006). Also, DC-SIGNR, which is 77% homologous to DC-SIGN, displays a similar specificity, except for the Lex epitope which is only recognized by DC-SIGN (Appelmelk et al., 2003; van Kooyk et al., 2003; van Die et al., 2003; Guo et al., 2004; van Liempt et al., 2004). The hallmark of carbohydrate binding to C-type lectins is the primary interaction between the principal Ca2+ and the vicinal hydroxyl groups of a pyranose ring. Further substrate specificity comes from secondary interactions between the carbohydrate and CRD-specific amino acids.
3.1. DC-SIGN binding to high-Man oligosaccharides
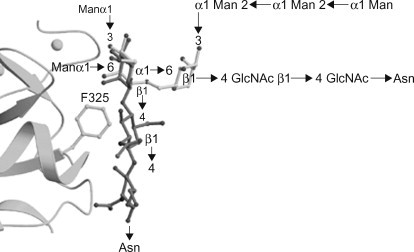
Solid-phase competition and binding assays have shown that DC-SIGN and DC-SIGNR interact with N-linked high-Man structures (Feinberg et al., 2001, Feinberg et al., 2007; Guo et al., 2004; van Liempt et al., 2006). Both proteins show the highest apparent affinity towards structures containing nine mannosyl residues, i.e. Man9GlcNAc2 (where GlcNAc, N-acetyl-d-glucosamine) (Figure 34.2(A) . Co-crystallization experiments have shown that the interaction between DC-SIGN/DC-SIGNR and high-Man structures is mediated by vicinal, equatorial 3- and 4-OH groups of internal mannosyl residues (Feinberg et al., 2001, Feinberg et al., 2007; Guo et al., 2004) (Figure 34.3A) . The preference for internal residues is unusual since most Man-binding lectins (e.g. MR and MBL) recognize terminal residues (Weis et al., 1992; Hitchen et al., 1998). In the first crystal structure that was published, DC-SIGN and DC-SIGNR were co-crystallized with the pentasaccharide Man3GlcNAc2; β-GlcNAc-(1→2)-α-Man-(1→3)-[β-GlcNAc-(1→2)-α-Man-(1→6)-]Man (Feinberg et al., 2001) (see Figure 34.2, Figure 34.3. The structures revealed that the interaction with the principal Ca2+ was mediated by the 3- and 4-OH groups of the α-(1→3)-linked mannosyl residue (Man2) (see Figure 34.3A; see Figure 34.2B for residue numbering). For DC-SIGNR, secondary interactions were mediated by phenylalanine residue 325 (Phe325) and serine residue 372 (Ser372) [Phe313 and Ser360 in DC-SIGN], in which Phe325 was located in the crevice between Man3 and Man4 and formed van der Waals contacts with Man3, and Ser372 participated in a hydrogen-bonding network involving both Man3 and Man4 (see Figure 34.3B). The structural information was used to obtain insight into the selectivity of DC-SIGN and DC-SIGNR for high-Man glycans. Superimposing the two distinct α-Man-(1→3)-[α-Man-(1→6)-]Man moieties present in Man9GlcNAc2 (see Figure 34.2A; light and dark grey boxes, respectively) on the equivalent portions of the oligosaccharide complexes in DC-SIGNR revealed that the β-(1→4)-linked GlcNAc of the inner tri-Man branch point (see Figure 34.2A; dark grey box) clashed with Phe325, whereas the outer tri-Man branch point (see Figure 34.2A; light grey box) did not (Feinberg et al., 2001) (Figure 34.4 ). Therefore, although DC-SIGN and DC-SIGNR recognize the α-Man-(1→3)-[α-Man-(1→6)-]Man trisaccharide, they can only do so when the central Man is linked in an α-anomeric conformation, a feature only found in high-Man structures. Taken together, these data demonstrated that, besides the principal Ca2+, DC-SIGN and DC-SIGNR harbour a secondary carbohydrate binding site (formed by Phe313 and Ser360 in DC-SIGN and Phe325 and Ser372 in DC-SIGNR) which makes additional contacts with the carbohydrate and explains the increased affinity towards higher order Man structures, as compared to Man alone. Interestingly, it was recently shown that DC-SIGN is able to bind an α-Man-(1→2)-Man disaccharide (either by itself or as part of a Man6 structure) independently of the α-Man-(1→3)-[α-Man-(1→6)-]Man trisaccharide (Feinberg et al., 2007). This interaction encompassed not only the principal Ca2+ but also included a valine residue at position 351. This result suggests that the observed affinity enhancement towards oligosaccharides that besides a tri-Man core also contain terminal α-Man-(1→2)-Man groups (e.g. Man9GlcNAc2) is due to multiple binding modes to the CRD, which provide both additional contacts (mediated by Val351) and a statistical (entropic) enhancement of binding (Feinberg et al., 2007).
Figure 34.2.
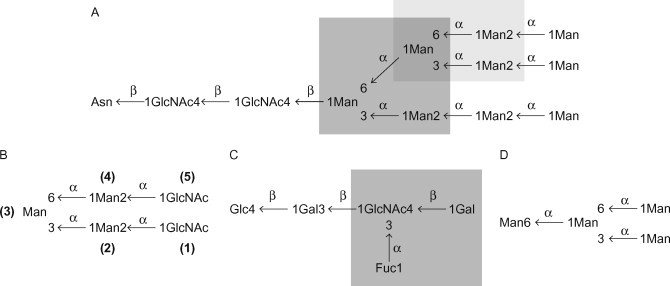
Schematic representations of mannose- (Man-) containing structures that interact with DC-SIGN and DC-SIGNR. (A) the N-linked high-mannose (Man) structure Man9GlcNAc2; the inner branched trimannose structure α-Man-(1→3)-[α-Man-(1→6)-]Man is indicated by the dark grey box, whereas the outer tri-Man structure is indicated by the light grey box; (B) the pentasaccharide Man3GlcNAc2 that has been co-crystallized with DC-SIGN and DC-SIGNR (residues are numbered as mentioned in the text); (C) the lacto-N-fucopentaose III pentasaccharide; and (D) the Man4 oligosaccharide structures that were co-crystallized with DC-SIGN. For lacto-N-fucopentaose III, the Lex structure is highlighted by the grey box. Abbreviations: Asn, asparagine; Fuc, l-fucose; Man, d-mannose; Gal, d-galactose; Glc, d-glucose; GlcNAc, N-acetyl-d-glucosamine.
In part adapted, with permission, from Feinberg et al. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. © AAAS.
Figure 34.3.

Co-crystallization of Man3GlcNAc2 with DC-SIGN and DC-SIGNR. (A) Interaction of the α-(1→3)-linked branch of Man3GlcNAc2 with the carbohydrate recognition domain (CRD) of DC-SIGN. For clarity, some of the sugar residues are shown schematically. Large spheres represent Ca2+ ions. The Ca2+ coordination bonds are shown as solid black lines; hydrogen bonds as dashed lines. Important residues are numbered. Of note, the terminal GlcNAc1 interacts with the principal Ca2+ ion of another DC-SIGN CRD (Note the second CRD is not shown). (B) Interaction of the α-(1→6)-linked branch of Man3GlcNAc2 with the CRD of DC-SIGNR. Hydrogen bonds are shown as dashed lines and important residues are numbered. Abbreviations: GlcNAc, N-acetyl-d-glucosamine; Man, d-mannose.
Derived, with permission, from Feinberg et al. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. © AAAS.
Figure 34.4.
Ribbon diagram of the carbohydrate recognition domain (CRD) of DC-SIGNR with the Phe325 side chain in a ball-an-stick representation. The inner (in dark grey) and outer (in light grey) branched trimannose structures of Man9GlcNAc2 were superimposed on the central (reducing) mannose of the Man3GlcNAc2 structure that was co-crystallized with DC-SIGNR. The model was made by using average torsion angle values, with some small adjustments to overlay the structures precisely. Abbreviations: Asn, asparagine; GlcNAc, N-acetyl-d-glucosamine; Man, d-mannose.
Derived, with permission, from Feinberg et al. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. © AAAS.
3.2. DC-SIGN binding to branched Fuc structures
The first observations that DC-SIGN binds branched fucosylated structures were made in studies examining the interaction between DC-SIGN and surface glycans of human pathogens (Appelmelk et al., 2003; van Die et al., 2003). In one of these studies, it was observed that the interaction between Schistosoma mansonisoluble egg antigens (SEAs) and DC-SIGN could be inhibited by antibodies recognizing Lex, β-Gal-(1→4)-[α-Fuc-(1→3)-]GlcNAc and N-acetyl-d-galactosamine- (GalNAc-) containing LDNF, β-GalNAc-(1→4)-[α-Fuc-(1→3)-]GlcNAc, epitopes (both present on SEAs), thereby suggesting that these structures bind to DC-SIGN (van Die et al., 2003). This was supported by the observation that DC-SIGN–Fc specifically bound to polyvalent neo-glycoconjugates harbouring the Lex epitope. In addition, it was shown that DC-SIGN binds to related Le epitopes, such as: Lea, β-Gal-(1→3)-[Fuc-α-(1→4)-]GlcNAc; Leb, α-Fuc-(1→2)-β-Gal-(1→3)-[Fuc-α-(1→4)-]GlcNAc; and Ley, α-Fuc-(1→2)-β-Gal-(1→4)- [α-Fuc-(1→3)-]GlcNAc but not to sulfo- or sialyl-Lex (Appelmelk et al., 2003). Similar to DC-SIGN, DC-SIGNR can recognize Lea, Leb and Ley epitopes (van Liempt et al., 2004). However, DC-SIGNR does not bind to the Lex epitope (Guo et al., 2004; van Liempt et al., 2004). Insight into the molecular mechanism underlying this differential recognition was provided when the CRD of DC-SIGN was co-crystallized with the Lex-containing pentasaccharide lacto-N-fucopentaose III (Guo et al., 2004) (see Figure 34.2, Figure 34.5. The structure revealed that the interaction between Lex and the principal Ca2+ was mediated by the α-(1→3)-linked Fuc residue. Because in Fuc the 3-OH group is in equatorial and the 4-OH group is in axial conformation, the Fuc ring was tipped compared to the mannosyl residue (for Man both the 3- and 4-OH groups are in equatorial conformation) in a structure of DC-SIGN co-crystallized with a Man4 oligosaccharide (Guo et al., 2004) (see Figure 34.2, Figure 34.5). As a consequence of this orientation, the Fuc ring is close to Val351, which forms tight van der Waals contacts with its 2-OH group. In addition, the terminal Gal residue binds to a secondary binding site encompassing Glu358, Asp367, Lys368, Leu371 and Lys373 (where Glu, glutamicacid; Asp, aspartic acid; Lys, lysine; Leu, leucine)(Guo et al., 2004).
Figure 34.5.
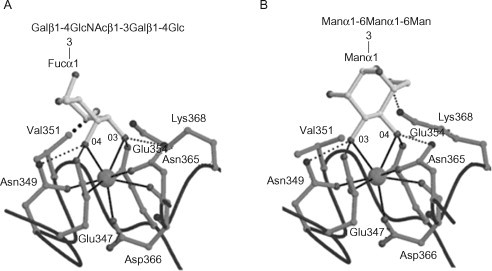
Co-crystallization of lacto-N-fucopentaose III and a Man4 oligosaccharide with DC-SIGN. Close-up of (A) the α-(1→3)-linked fucose residue of lacto-N-fucopentaose III or (B) the terminal, non-reducing α-(1→3)-linked mannose residue of the Man4 oligosaccharide in the primary binding site of DC-SIGN. The large spheres represent the principal Ca2+ ions. The Ca2+ coordination bonds are shown as solid black lines, hydrogen bonds as thin dashed lines and van der Waals contacts as thick dashed lines. Important residues are numbered. Abbreviations: Asn, asparagines; Asp, aspartic acid; Fuc, l-fucose; Lys, lysine; Man, d-mannose; Gal, d-galactose; Glc, d-glucose; GlcNAc, N-acetyl-d-glucosamine; Glu, glutamic acid; Val, valine.
Reproduced, with permission, from Guo et al. (2004) Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11, 591–598.
Interestingly, the Val351 residue is substituted for a serine residue (Ser363) in DC-SIGNR, eliminating the van der Waals contact with the 2-OH group. This feature, together with a subtle difference in ligand orientation due to differences in amino acid sequence can explain the inability of DC-SIGNR to bind Lex. This is supported by the observation that substituting Ser363 in DC-SIGNR for a valine residue enables it to bind to Lex epitopes (Guo et al., 2004; van Liempt et al., 2004). The reason why DC-SIGNR does not bind Lex but is able to interact with closely related structures such as Lea and Ley remains unclear and probably involves differences in stericity and/or bulkiness of the corresponding structures. Further co-crystallization experiments of DC-SIGN and DC-SIGNR with other Le antigens are needed to obtain insight into the mechanism underlying this differential recognition.
4. In Vivo Function and Role in Dendritic Cell Biology of DC-SIGN
Dendritic cells are professional antigen presenting cells crucial for mounting successful immune responses against invading pathogens and for maintaining immune tolerance towards endogenous ligands. The iDCs originate from the bone marrow where they develop from both myeloid- and lymphoid-committed progenitors (Wu and Liu, 2007). After entering the blood stream, iDCs extravasate into peripheral tissues where they continuously sample their environment by capturing antigens and subsequently presenting them on major histocompatibility (MHC) complexes. Upon pathogen encounter, iDCs receive activation signals that trigger their maturation and stimulate migration into secondary lymphoid organs where they interact with naïve T-cells and initiate an adaptive immune response. Both DC maturation and migration are tightly controlled processes dictated by a variety of cytokines, chemokines and adhesion molecules. Adhesion molecules are not only important for facilitating cellular interactions involved in DC migration, i.e. DC–endothelial cell interactions, but also play a role in establishing DC–T-cell contacts that enable MHC scanning and T-cell receptor activation.
4.1. DC-SIGN facilitates DC migration from the blood into tissues
Migration of iDCs into surrounding tissues is an important process that is pivotal for the successful detection and eradication of invading pathogens. In recent years, the molecular mechanisms underlying DC migration have become increasingly clear and several molecules are now known to be involved, including various C-type lectins such as the MR, the selectins and DC-SIGN (Irjala et al., 2001; Geijtenbeek et al., 2002a, Geijtenbeek et al., 2004). Indeed, DC-SIGN was shown to mediate the rolling and adhesion of iDCs on vascular endothelial cells by interacting with ICAM-2 (Geijtenbeek et al., 2000a). Furthermore, the DC-SIGN–ICAM-2 interaction was shown to facilitate the transmigration of iDCs across both resting and activated endothelia (Geijtenbeek et al., 2000a). Since ICAM-2 expression is constitutive and does not depend on endothelial activation (Nortamo et al., 1991), DC-SIGN–ICAM-2 interactions probably play a central role in homeostatic extravasation of iDCs into surrounding tissues. Due to its heavy glycosylation, it has long been unclear what particular ICAM-2 structure is recognized by DC-SIGN. Although high-Man structures were initially suspected (Jimenez et al., 2005), it was recently demonstrated that the interaction is primarily dependent on Ley epitopes present on ICAM-2 (Garcia-Vallejo et al., 2008).
4.2. DC-SIGN mediates DC–T-cell interactions
After receiving activation signals, iDCs become mature and migrate to secondary lymphoid organs where they interact with T-cells and stimulate an adaptive immune response. The initial DC–T-cell contact is transient and allows for the rapid scanning of MHC complexes by T-cells. Studies aimed at identifying molecules important in this process suggested the involvement of ICAM-3 (Hauss et al., 1995; Starling et al., 1995). Initially, it was thought that T-cell ICAM-3 interacted with leukocyte function antigen-1 (LFA-1) on DCs (Hauss et al., 1995), however, later on, it was shown that the affinity of this interaction is low and that the actual DC counter-receptor is DC-SIGN (Geijtenbeek et al., 2000b). It was demonstrated that DC-SIGN binds ICAM-3 with high affinity and forms the major ICAM-3 receptor on DCs. Monoclonal antibodies blocking DC-SIGN inhibited DC–T-cell clustering and prevented DC-dependent proliferation of resting T-cells. Furthermore, it was shown that the DC-SIGN-ICAM-3 interaction is transient, which allows for the rapid screening of MHC-peptide complexes (Geijtenbeek et al., 2000b). Although these data seem strong and the interaction between DC-SIGN and ICAM-3 has been confirmed independently (Geijtenbeek et al., 2002b; Su et al., 2004; Jimenez et al., 2005), a potential problem may be that these studies were performed using recombinant proteins. It is well known that protein glycosylation is not only dependent on the proteins themselves but is also influenced by the nature of the cell line in which they are expressed and the cell culture conditions that are used (Brooks, 2006; Devasahayam, 2007). The possibility that the utilization of recombinant proteins may have led to misinterpretation was raised in a recent paper in which the interactions between DC-SIGN–Fc and ICAM-3 molecules directly purified from blood leukocytes were investigated (Bogoevska et al., 2007). Unexpectedly, this study found that DC-SIGN–Fc could only bind to ICAM-3 molecules that were derived from granulocytes and not to ICAM-3 that was isolated from monocytes, B-cells and, most importantly, T-cells. Moreover, it was found that the interaction was dependent on Lex and could be abolished by pretreating ICAM-3 with fucosidase III (Bogoevska et al., 2007). The presence of Lex epitopes on ICAM-3 was confirmed using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry and an antibody direct against Lex, which only recognized granulocyte ICAM-3 and not the ICAM-3 of other cell types. Surprisingly, these observations do not support the earlier finding that ICAM-3 plays an important role in DC–T-cell interactions (Geijtenbeek et al., 2000b). Furthermore, they contradict an earlier study in which it was shown that native, neutrophil-derived ICAM-3 binds DC-SIGN–Fc with poor affinity (van Gisbergen et al., 2005a). Possible explanations for these conflicting results may be:
-
(i)
the genetic variability between different donors;
-
(ii)
differences in ICAM-3 preparations due to alternative isolation procedures (immunoprecipitation versus affinity chromatography); and
-
(iii)
structural differences between the DC-SIGN–Fc constructs that were used.
Whereas Bogoevska et al. (2007) used an N-terminal DC-SIGN–Fc fusion protein produced in human embryonic kidney 293 cells, van Gisbergen et al. (2005a) used a C-terminal DC-SIGN–Fc fusion protein prepared from Chinese hamster ovary K1 cells. That the origin of recombinant soluble lectin can indeed determine specificity was recently shown for the interaction of the NK-cell lectin NKp30 with heparan sulfate (Hershkovitz et al., 2008). Furthermore, van Gisbergen et al. (2005a) did not include a control for the amount of ICAM-3 that was present on their blots. Nevertheless, the observation that the interaction of T-cells with iDCs can be blocked by monoclonal antibodies against DC-SIGN (Geijtenbeek et al., 2000b) suggests that DC-SIGN plays an important role in early DC–T-cell contacts. The question whether these interactions are indeed dependent on ICAM-3 or, as has now been suggested, are mediated by different molecules remains open and awaits further investigation.
4.3. Role of DC-SIGN in DC-neutrophil interactions
Neutrophils are phagocytic cells that play an important role in innate immunity by engulfing and killing extracellular pathogens. Upon infection, neutrophils infiltrate inflamed tissues where they kill pathogens through phagocytosis and the release of antimicrobial compounds. Although primarily beneficial, neutrophil-derived oxidants, proteinases, cationic peptides and reactive oxygen species can damage surrounding tissues (Moraes et al., 2006). For this reason, neutrophil turnover is a tightly controlled process which is dependent on a fine balance between pre- and anti-apoptotic signals (Walker et al., 2005). In addition, neutrophils play a role in adaptive immune responses not only by recruiting additional immune cells to the site of infection but also by directly influencing their activity. More recently, it was shown that neutrophils can directly interact with iDCs (van Gisbergen et al., 2005a). It was demonstrated that this interaction is dependent on DC-SIGN (van Gisbergen et al., 2005a). Up until now, three potential neutrophil DC-SIGN-ligands have been identified. Besides ICAM-3, also Mac-1 (CD11b/CD18) and the carcinoembryonic antigen-related cellular adhesion molecule (CEACAM) 1 were shown to bind DC-SIGN (van Gisbergen et al., 2005a, van Gisbergen et al., 2005b; Bogoevska et al., 2006, Bogoevska et al., 2007). In all cases, binding of DC-SIGN to its neutrophil counter-receptor was dependent on Lex epitopes that were expressed on these proteins. What effect DC-SIGN binding has on the neutrophil response is currently not well understood, however, it is known that ICAM-3, Mac-1 and CEACAM1 are involved in the regulation of neutrophil apoptosis (Yan et al., 2004; Singer et al., 2005; Kessel et al., 2006). Therefore, DC-SIGN-dependent DC–neutrophil interactions may affect neutrophil survival (Ludwig et al., 2006). This hypothesis is supported by the finding that the interaction of DCs with neutrophils prevents the downregulation of several markers, i.e. CD13, CD15, CD16 and Mac-1, on neutrophils (Megiovanni et al., 2006). Downregulation of these markers is associated with increased neutrophil apoptosis (Dransfield and Rossi, 2004). Besides modulating neutrophil responses, DC-SIGN–neutrophil interactions also influence the DCs themselves. This was demonstrated by the observation that neutrophil-induced DC maturation provokes a strong T-helper-1 cell (Th1-) polarized DC response (van Gisbergen et al., 2005a). Collectively, these findings illustrate the importance of DC-SIGN in DC–neutrophil interactions. Furthermore, they show that these interactions influence both the neutrophil and the DC.
4.4. DC-SIGN functions as an endocytic antigen receptor
The C-type lectins are well known for their capability to recognize antigens and mediate their uptake by antigen presenting cells. Besides the macrophage MR (Apostolopoulos and McKenzie, 2001), also DEC-205 (Mahnke et al., 2000), BDCA-2 (Dzionek et al., 2001), and DC-SIGN (Engering et al., 2002a; Schjetne et al.,2002) have been shown to perform such a function. By using anti-DC-SIGN monoclonal antibodies, it was demonstrated that DC-SIGN quickly internalizes upon antigen binding and subsequently traffics to late endosomal or lysosomal compartments where the complexes are degraded, loaded onto MHC complexes and presented to T-cells (Engering et al., 2002a). In addition, DC-SIGN has been shown to mediate the recognition and uptake of various pathogens including human immunodeficiency virus (HIV)-1 and Leishmania amastigotes (Kwon et al.,2002; Colmenares et al., 2002). Notably, DC-SIGN endocytosis is dependent upon the di-leucine motif in its cytoplasmic tail (Engering et al.,2002a) (see Figure 34.1A). Whereas wild-type DC-SIGN was efficiently taken up, a mutated form, in which the leucines were replaced by alanines, endocytosed much less efficiently (Engering et al., 2002a). In addition, DC-SIGN harbours a tri-acidic cluster: EEE (in one-letter amino-acid code) (see Figure 34.1A). For DEC-205, it has been shown that a comparable tri-acidic motif (EDE instead of EEE) mediates targeting to late endosomal compartments (Mahnke et al., 2000). Thus, in conclusion, DC-SIGN not only functions as an adhesion receptor but also mediates the uptake and presentation of pathogen-derived antigens by antigen presenting cells. While these functions suggest a primary role in host defence mechanisms, an increasing amount of evidence suggests that DC-SIGN–pathogen interactions may also be part of pathogenic strategies to increase the efficacy of infection and/or escape immune surveillance by modulating host immune responses.
5. Pathogens Target DC-SIGN to Subvert Host Immune Responses
As discussed above, DC-SIGN functions as an antigen receptor that mediates the recognition and uptake of a wide variety of pathogens (see Table 34.1). The first pathogen that was shown to interact with DC-SIGN is HIV-1 (Geijtenbeek et al., 2000c). It was demonstrated that binding of HIV-1 is mediated by the interaction between DC-SIGN and the HIV-1 glycoprotein gp120. Furthermore, it was shown that HIV-1 binding by DCs facilitates infection of HIV-1 permissive cells in trans (Geijtenbeek et al., 2000c). Later on, several other viral pathogens were also shown to interact with DC-SIGN, demonstrating its function as a broad viral receptor. The interaction of DC-SIGN with viral pathogens and its role in viral infectivity has been the subject of many studies. Several high-quality reviews on DC-SIGN–virus interactions have recently appeared, including ones discussing the interaction of DC-SIGN with HIV-1 (Lekkerkerker et al., 2006; Wu and KewalRamani, 2006), hepatitis C virus (Cocquerel et al., 2006), Ebola virus (Baribaud et al., 2002) and severe acute respiratory syndrome (SARS) corona virus (Chen and Subbarao, 2007). For this reason, DC-SIGN-virus interactions will not be discussed in further detail. Rather we will focus on the interaction of DC-SIGN with another important class of pathogens, i.e. bacterial pathogens. In the next section, we will first discuss the ability of mycobacterial species to target DC-SIGN and thereby subvert DC functioning. After this, we continue with another pathogen, i.e. Helicobacter pylori, and see how this bacterium specifically modulates the Th1/Th2 balance through phase-variable interactions between its lipopolysaccharide (LPS) and DC-SIGN.
5.1. Mycobacteria target DC-SIGN to subvert DC functioning
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is a major cause of death worldwide. The bacterium is transmitted through aerosols spread by people suffering from active clinical disease. After inhalation, M. tuberculosis infects alveolar macrophages in which it is able to persist for extensive periods of time. Normally, the infected macrophages are contained within so-called granulomas, however, in a substantial number of cases (≈10%), the bacterium is released from its containment and causes active disease (Russell, 2007). To date, the only licensed TB vaccine is the so-called Bacille Calmette-Guérin (BCG) vaccine. Although effective against disseminated TB in children, it does not protect well against pulmonary TB later in life. Moreover, the prevalence of multiple- and extensive drug-resistant M. tuberculosis strains increases each year.
The hallmark of mycobacterial disease is the bacterium’s ability to persist in host tissues for many years. This feature, which is also referred to as latency, is dependent on at least two different processes. The first one is the ability of the bacterium to inhibit phago–lysosome fusion. Normally, ingested bacteria are contained within phagosomes which later on fuse with lysosomes leading to bacterial destruction and subsequent presentation on MHC complexes. However, pathogenic mycobacteria interfere with this process by actively blocking phago–lysosome fusion thereby creating a unique niche in which they are able to survive for many years (Rohde et al., 2007). For the past decades, the central dogma has been that pathogenic mycobacteria exclusively reside within this phagosomal compartment until they are released from the cell and cause active disease. However, recently, this view was challenged by the observation that M. tuberculosis-containing phagosomes rapidly fused with lysosomes in both monocyte-derived DCs and macrophages (van der Wel et al., 2007). Yet, at day 2 post infection, M. tuberculosis was able to escape from the phago–lysosomes into the cytosol where they were able to replicate (van der Wel et al., 2007). This same phenomenon was observed for Mycobacterium leprae but not for the vaccine strain Mycobacterium bovis BCG or for heat-killed mycobacteria, suggesting that the process is specific for pathogenic mycobacteria. Although these findings contradict earlier observations and await independent confirmation, they may have important implications for the current view on host–mycobacterial interactions.
A second feature that supports the ability of pathogenic mycobacteria to cause chronic infections is their capacity to suppress host immune responses. To this end, DCs form an interesting target due to their central role in the induction of adaptive immunity. Although early data already indicated that mycobacteria influence DC functioning, the underlying mechanisms remained poorly understood. Major progress into this field was made when it was found that the interaction between M. tuberculosis and DCs is almost exclusively dependent on the binding to DC-SIGN (Geijtenbeek et al., 2003; Tailleux et al., 2003) (see also Chapter 9). Although DCs express a wide variety of C-type lectins, such as the MR and dectin-1, only antibodies directed against DC-SIGN could block the Mycobacterium–DC interaction (Tailleux et al., 2003). Comparable results were obtained with the M. bovis BCG vaccine strain (Tailleux et al., 2003; Geijtenbeek and van Kooyk, 2003).
In an effort to identify the ligand responsible for binding to DC-SIGN, it was found that binding to M. tuberculosis could almost completely be inhibited by pre-incubating DC-SIGN with Man-capped lipoarabinomannan (ManLAM), a major glycolipid of the M. tuberculosis cell wall, suggesting that this component forms an important ligand for DC-SIGN (Tailleux et al., 2003). Moreover, it was shown that the interaction of ManLAM with DC-SIGN was dependent on the terminal Man caps as AraLAM, an uncapped form of ManLAM, did not bind to DC-SIGN (Geijtenbeek et al., 2003). Later on, these findings were confirmed by the demonstration that DC-SIGN specifically interacts with neo-glycoconjugates that resemble the Man-cap of ManLAM (Koppel et al., 2004). Furthermore, it was shown that a reduction of the number of mannosyl residues in the cap-derived neo-glycoconjugates leads to a decreased affinity for DC-SIGN (Koppel et al., 2004). Interestingly, ManLAM is exclusively found in pathogenic, slow-growing mycobacteria, whereas AraLAM is only found in avirulent, fast-growing species, suggesting that ManLAM-DC-SIGN interactions may be important for mycobacterial pathogenesis. These data, together with the finding that also PI-LAM, a phosphoinositide-capped form of LAM, poorly inhibits DC-SIGN–M. tuberculosisinteractions, led to the hypothesis that DC-SIGN can discriminate between mycobacterial species through the recognition of the Man caps on LAM (Maeda et al., 2003). However, later on, this view was found to be too simplistic as it was demonstrated that not all mycobacterial species that contain ManLAM are bound by DC-SIGN (Pitarque et al., 2005). This was not due to intrinsic differences between the different ManLAMs, as it was shown that the purified ManLAMs, also the ones from the species that did not bind DC-SIGN, could all efficiently block DC-SIGN–M. tuberculosis interactions (Pitarque et al.,2005). Furthermore, experiments addressing the cell-surface exposure of ManLAM showed that between species only minor differences exist (Pitarque et al., 2005). These data strongly suggested that ManLAM was not the only DC-SIGN ligand present on mycobacteria and further investigations led to the discovery of at least four additional ligands: lipomannan, Man-capped arabinomannan and two mannosylated glycoproteins (19 and 45 kDa antigens) (Pitarque et al., 2005). In a later study, also phosphatidylinositol-mannosides were shown to bind to DC-SIGN (Torrelles et al., 2006).
The strongest evidence that the presence of Man caps on LAM is not essential for the binding of mycobacteria to DC-SIGN was provided in a study using Mycobacterium marinum and M. bovis BCG strains devoid of Man caps on LAM (Appelmelk et al., 2008). It was demonstrated that the mutant strains bound as efficiently to DC-SIGN as the wild-type strains. Furthermore, the interaction could still be blocked with mannan. In addition, the mutants did not show any significant differences in in vivo survival and induced similar cytokine profiles (Appelmelk et al., 2008). The only difference observed was that the phagocytosis of the capless M. marinum strain by macrophages was slightly reduced and that the mutant induced somewhat more phagosome–lysosome fusion, which is consistent with the earlier finding that ManLAM inhibits this process (Fratti et al., 2003; Hmama et al., 2004). Overall, this study demonstrated that the Man caps of LAM do not dominate the Mycobacterium–host interaction. This finding was unexpected since ManLAM was not only shown to bind to DC-SIGN but was also known to modulate DC cytokine secretion by suppressing the production of IL-12 and/or upregulating the production of IL-10, thereby inhibiting Th1-type immune responses (Nigou et al., 2001; Geijtenbeek et al., 2003). Interestingly, Geijtenbeek et al. (2003) observed that ManLAM, but not AraLAM, induces IL-10 secretion in LPS-primed DCs. By using monoclonal antibodies, they demonstrated that the phenomenon was dependent on DC-SIGN, suggesting that DC-SIGN ligation can modulate TLR responses (Geijtenbeek et al., 2003).
Recently, the molecular signalling pathway underlying this modulatory activity was identified (Gringhuis et al., 2007). It was demonstrated that pathogens target DC-SIGN to activate the serine/threonine kinase Raf-1, which subsequently leads to acetylation of the nuclear factor-kappaB (NF-κB) subunit p65, but only after NF-κB has been activated via TLRs. Acetylation of p65 both prolonged and increased IL-10 transcription to enhance the DCs anti-inflammatory cytokine responses (Gringhuis et al., 2007). These results demonstrate that the role of DC-SIGN in host–pathogen interactions can be interpreted in two ways. On the one hand, DC-SIGN forms an important pathogen receptor that mediates the uptake and destruction of a large panel of pathogenic microorganisms. Yet, on the other hand, pathogens can also exploit DC-SIGN to modulate DC responses and thereby subvert host immunity. The potential importance of DC-SIGN ligation during host infection is illustrated by the observation that microorganisms, such as mycobacteria, may express a large number of DC-SIGN ligands. Furthermore, pathogens may actively induce the expression of DC-SIGN. Interestingly, in the case of M. tuberculosis, it has been shown that in patients with TB, up to 70% of alveolar macrophages express DC-SIGN. By contrast, the lectin was hardly detected in alveolar macrophages from healthy individuals or in patients with unrelated lung pathologies (Tailleux et al., 2005). Moreover, promoter polymorphisms that influence DC-SIGN expression have been associated with an altered susceptibility to mycobacterial infections (Barreiro et al., 2006; Vannberg et al., 2008).
5.2. H. pylori modulates Th1/Th2 polarization through interactions with DC-SIGN
H. pylori is a common human pathogen that has persistently colonized about 50% of the total human population. Colonization typically occurs early in life and may persist during the host’s entire lifetime. In most cases, the infection is asymptomatic, however, in about 10% of the cases, the infection leads to disease, which can range from relatively mild gastritis and peptic ulcers to life-threatening diseases such as gastric cancer (Ernst and Gold, 2000; Makola et al.,2007). H. pylori expresses a wide variety of virulence factors including LPS. In general, LPS is an amphiphilic molecule that is located in the outer membrane of Gram-negative bacteria. It consists of three distinct domains: lipid A, a core oligosaccharide and an O-antigen (Raetz and Whitfield, 2002) (see Chapters 3 and 4Chapter 3Chapter 4). Although LPSs are considered to have a strong immunostimulatory activity which is primarily dependent on the interaction between lipid A and TLR-4 (Palsson-McDermott and O’Neill, 2004), H. pylori lipid A is unusual in that it contains a variety of modifications that abrogate the interaction with TLR-4, thereby lowering theendotoxic activity of the LPS (Moran, 2007). The H. pylori core oligosaccharide consists of an inner core of a 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) moiety substituted with a linear heptose tetrasaccharide and an outer core encompassing Gal and glucose residues (Aspinall et al., 1996; Aspinall and Monteiro, 1996). The O-antigen is linked to the heptose core and is composed of a poly-(N-acetyl-β-lactosamine) chain decorated at somepositions with l-Fuc residues to produce internal Lex determinants with terminal Lex or Ley moieties (Aspinall et al., 1996; Aspinall and Monteiro, 1996). Furthermore, some strains may express Lea, Leb, Lec and sialyl-Lex units, as well as H-1 and blood groups A and B antigens, giving rise to a large variation in LPS-core composition (Moran, 2008). Importantly, Lex and Ley expression is a common property of H. pylori strains and it is found in 80–90% of all cases. Yet, expression of Le epitopes can vary within a single strain as a consequence of the phase variable expression of the responsible fucosyltransferases, i.e. FutA, FutB and FutC (Wang et al., 2000; Bergman et al., 2006; Sanabria-Valentin et al., 2007). The frequency of “on–off” switching of Le antigen expression has been reported to be between 0.2 and 0.5% (Appelmelk et al., 1998). Biologically, Le epitope expression is thought to mediate the evasion of host immune responses and influence bacterial colonization and adhesion (Moran, 2008). Furthermore, Le antigen expression has been shown to mediate the binding of H. pylori to DC-SIGN (Bergman et al., 2004). Whereas strains harbouring Lex and Ley epitopes strongly bound to DC-SIGN, strains devoid of these structures did not (Bergman et al., 2004). Interestingly, it was demonstrated that the Le-dependent interaction with DC-SIGN inhibited Th1 responses by inducing the production of IL-10 (Bergman et al., 2004). Thus, since Le expression is phase variable, a typical H. pylori population will contain a mixture of DC-SIGN-binding and non-binding bacteria. Bacteria unable to bind DC-SIGN will primarily induce a Th1-type response. Yet, this Th1 polarization is counterbalanced by bacteria that target DC-SIGN, thereby leading to a mixed Th1/Th2 response. It is hypothesized that the induction of a mixed response promotes the establishment of chronic infections. This is supported by the observation that people suffering from chronic gastritis display a combined secretion of both Th1 and Th2 cytokines (D’Elios et al., 2005). Furthermore, it is known that Th1 polarization of H. pylori-specific T-cell responses is associated with more severe disease (D’Elios et al., 2005).
6. Conclusions
Ever since its discovery, DC-SIGN has received major scientific attention. It is now clear that the biological role of DC-SIGN is two-fold. On the one hand, DC-SIGN fulfils important functions necessary for the induction of successful immune responses that are essential for the clearance of microbial infections. Yet, on the other hand, pathogens may also exploit DC-SIGN to modulate DC functioning thereby skewing the immune response and promoting their own survival. Currently, a lot is known about the structure and carbohydrate specificity of DC-SIGN and crystallographic studies have provided us with detailed insights into the molecular mechanisms underlying DC-SIGN–carbohydrate interactions. However, much less is known about the role of DC-SIGN during in vivo infection. Obviously, there are good indications that suggest it plays an important role, however, these are mostly based upon in vitro experiments. Hence, one important goal is to obtain insight into the role of DC-SIGN during infection and see how it functions within the complexity of the immune system.
To enlighten this issue, the use of appropriate animal models seems essential. Nevertheless, one major problem is that, although DC-SIGN homologues can be found in many organisms, their carbohydrate specificity, expression pattern and immunological function may vary significantly. One example is the murine model. Like humans, mice express a set of DC-SIGN-like molecules, i.e. mDC-SIGN and mSIGNR1-4, of which some (mSIGNR1 and mSIGNR3) share a carbohydrate specificity similar to human DC-SIGN (Galustian et al., 2004). Yet, unlike human DC-SIGN, mSIGR1 is not expressed by DCs and mSIGNR3 only at low levels (Koppel et al., 2005), suggesting that these molecules exhibit distinct functions and, thus, that the murine model is unsuitable for studying the role of human DC-SIGN. One possibility is to use primates as these animals possess DC-SIGN homologues that resemble both the specificity and expression pattern of human DC-SIGN. However, due to both ethical and technical difficulties their use may not be feasible. A second option is to make use of humanized model systems. By reconstituting mice with human immune cells or by heterologously expressing human DC-SIGN in murine strains, it may be possible to study DC-SIGN in in vivo relevant situations. Both these approaches have recently been applied to study the function of DC-SIGN (Kretz-Rommel et al., 2007; Schaefer et al., 2008).
Another important goal is to dissect further the molecular signalling cascades downstream of DC-SIGN. It is clear that DC-SIGN can interfere with TLR signalling. It has been shown that DC-SIGN directly influences the acetylation of NF-κB p65 via Raf-1. Nonetheless, it remains unclear whether this route is the only way by which DC-SIGN influences TLR-signalling or whether alternative regulatory mechanisms mayexist. Furthermore, it is unclear whether DC-SIGN can also influence unrelated signalling pathways. Moreover, it will be interesting to see whether distinct DC-SIGN ligands all function in a similar way.
The increasing knowledge on DC-SIGN has also led to some, potentially, interesting applications. One idea is that DC-SIGN can be used to target antigens specifically to DCs, thereby generating a more efficient immune response. The feasibility of such an approach was recently demonstrated when it was shown that glycan modification of the tumour antigen gp100 targets it to DC-SIGN and enhances DC-induced antigen presentation to T-cells (Aarnoudse et al., 2008). Furthermore, it has been shown that administration of anti-DC-SIGN antibodies fused with either tetanus toxoid peptides or keyhole limpet haemocyanin raised efficient T-cell responses without additional adjuvant requirements (Kretz-Rommel et al., 2007). Therefore, targeting of antigens to DC-SIGN may be a promising strategy to induce enhanced immune responses against both cancer and microbial antigens. Currently, the picture is emerging that pathogens target DC-SIGN specifically to suppress Th1 immunity, as exemplified by the observations that both mycobacterial ManLAM and H. pylori LPS suppress IL-12 secretion and/or induce IL-10 production by immune cells. In principle, this knowledge can be used to counteract the bacteria as the removal of DC-SIGN ligands may thus lead to improved Th1 responses and, thereby, enhance the efficacy of certain vaccines. One important vaccine to which this approach may apply is the BCG vaccine which is currently used to prevent M. tuberculosis infection. This vaccine is effective in preventing disseminated TB in children, but is poorly able to prevent pulmonary TB later in life. The general idea is that the BCG vaccine induces a sub-optimal immune response thereby abrogating protective immunity. As DC-SIGN seems to be involved in mycobacterial immunosuppression, it would be interesting to see whether the removal of mycobacterial DC-SIGN ligands can increase the potency of BCG vaccines.
Overall, the discovery of DC-SIGN has led to important insights into the interactions between pathogens and their hosts. Yet, important questions remain to be resolved (see Research Focus Box). Nevertheless, DC-SIGN forms an interesting target that may pave the way for the design of new therapeutic approaches against both microbial infections and cancer.
RESEARCH FOCUS BOX.
-
•
Why does DC-SIGNR bind to Lea and Ley but not to the closely related Lex structure?
-
•
How important is ICAM-3 in DC-SIGN-dependent DC and T-cell interactions?
-
•
What is the functional role of DC-SIGN splice variants?
-
•
How important is DC-SIGN ligation during in vivo infection?
-
•
Do all DC-SIGN ligands behave similarly in terms of induced downstream signalling cascades?
-
•
Does DC-SIGN ligation modulate other host cell responses than IL-10/IL-12 cytokine production?
-
•
How important are DC-SIGN-related polymorphisms for susceptibility to and outcome of microbial infections?
-
•
How feasible is the approach to target DC-SIGN for the development of novel therapeutics against microbial infections, cancer and, maybe, autoimmune diseases?
References
- Aarnoudse C.A., Bax M., Sanchez-Hernandez M., Garcia-Vallejo J.J., van Kooyk Y. Glycan modification of the tumor antigen gp100 targets DC-SIGN to enhance dendritic cell induced antigen presentation to T cells. Int. J. Cancer. 2008;122:839–846. doi: 10.1002/ijc.23101. [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V., McKenzie I.F. Role of the mannose receptor in the immune response. Curr. Mol. Med. 2001;1:469–474. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- Appelmelk B.J., Shiberu B., Trinks C. Phase variation in Helicobacter pylori lipopolysaccharide. Infect. Immun. 1998;66:70–76. doi: 10.1128/iai.66.1.70-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk B.J., van Die I., van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- Appelmelk B.J., den Dunnen J., Driessen N.N. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Aspinall G.O., Monteiro M.A. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2498–2504. doi: 10.1021/bi951853k. [DOI] [PubMed] [Google Scholar]
- Aspinall G.O., Monteiro M.A., Pang H., Walsh E.J., Moran A.P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 1996;35:2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- Baribaud F., Doms R.W., Pohlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets. 2002;6:423–431. doi: 10.1517/14728222.6.4.423. [DOI] [PubMed] [Google Scholar]
- Barreiro L.B., Neyrolles O., Babb C.L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS. Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro L.B., Neyrolles O., Babb C.L. Length variation of DC-SIGN and L-SIGN neck-region has no impact on tuberculosis susceptibility. Hum. Immunol. 2007;68:106–112. doi: 10.1016/j.humimm.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ali M., Barreiro L.B., Chabbou A. Promoter and neck region length variation of DC-SIGN is not associated with susceptibility to tuberculosis in Tunisian patients. Hum. Immunol. 2007;68:908–912. doi: 10.1016/j.humimm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Bergman M.P., Engering A., Smits H.H. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M., Del P.G., van Kooyk Y., Appelmelk B.J. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nat. Rev. Microbiol. 2006;4:151–159. doi: 10.1038/nrmicro1344. [DOI] [PubMed] [Google Scholar]
- Bleijs D.A., Geijtenbeek T.B., Figdor C.G., van Kooyk Y. DC-SIGN and LFA-1: a battle for ligand. Trends Immunol. 2001;22:457–463. doi: 10.1016/s1471-4906(01)01974-3. [DOI] [PubMed] [Google Scholar]
- Bogoevska V., Horst A., Klampe B., Lucka L., Wagener C., Nollau P. CEACAM1, an adhesion molecule of human granulocytes, is fucosylated by fucosyltransferase IX and interacts with DC-SIGN of dendritic cells via Lewis x residues. Glycobiology. 2006;16:197–209. doi: 10.1093/glycob/cwj057. [DOI] [PubMed] [Google Scholar]
- Bogoevska V., Nollau P., Lucka L. DC-SIGN binds ICAM-3 isolated from peripheral human leukocytes through Lewis x residues. Glycobiology. 2007;17:324–333. doi: 10.1093/glycob/cwl073. [DOI] [PubMed] [Google Scholar]
- Brooks S.A. Protein glycosylation in diverse cell systems: implications for modification and analysis of recombinant proteins. Expert. R. Proteomics. 2006;3:345–359. doi: 10.1586/14789450.3.3.345. [DOI] [PubMed] [Google Scholar]
- Cambi A., Figdor C.G. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell Biol. 2003;15:539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Cambi A., Koopman M., Figdor C.G. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Subbarao K. The immunobiology of SARS*. Annu. Rev. Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Cocquerel L., Voisset C., Dubuisson J. Hepatitis C virus entry: potential receptors and their biological functions. J. Gen. Virol. 2006;87:1075–1084. doi: 10.1099/vir.0.81646-0. [DOI] [PubMed] [Google Scholar]
- Colmenares M., Puig-Kroger A., Pello O.M., Corbi A.L., Rivas L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J. Biol. Chem. 2002;277:36766–36769. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- D’Elios M.M., Amedei A., Benagiano M., Azzurri A., Del P.G. Helicobacter pylori, T cells and cytokines: the “dangerous liaisons”. FEMS Immunol. Med. Microbiol. 2005;44:113–119. doi: 10.1016/j.femsim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Devasahayam M. Factors affecting the expression of recombinant glycoproteins. Indian J. Med. Res. 2007;126:22–27. [PubMed] [Google Scholar]
- Dransfield I., Rossi A.G. Granulocyte apoptosis: who would work with a “real” inflammatory cell? Biochem. Soc. Trans. 2004;32:447–451. doi: 10.1042/BST0320447. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- Drickamer K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999;9:585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Dzionek A., Sohma Y., Nagafune J. BDCA-2,a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East L., Isacke C.M. The mannose receptor family. Biochim. Biophys. Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Engering A., Geijtenbeek T.B., van Vliet S.J. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Engering A., Van Vliet S.J., Geijtenbeek T.B., van Kooyk Y. Subset of DC-SIGN+ dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood. 2002;100:1780–1786. doi: 10.1182/blood-2001-12-0179. [DOI] [PubMed] [Google Scholar]
- Ernst P.B., Gold B.D. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Mitchell D.A., Drickamer K., Weis W.I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Guo Y., Mitchell D.A., Drickamer K., Weis W.I. Extended neck regions stabilize tetramers of the receptors DC-SIGN and DC-SIGNR. J. Biol. Chem. 2005;280:1327–1335. doi: 10.1074/jbc.M409925200. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Castelli R., Drickamer K., Seeberger P.H., Weis W.I. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 2007;282:4202–4209. doi: 10.1074/jbc.M609689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D.J., Beranek M.C., Baenziger J.U. A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc. Natl. Acad. Sci. USA. 1998;95:2089–2093. doi: 10.1073/pnas.95.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti R.A., Chua J., Vergne I., Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA, 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galustian C., Park C.G., Chai W. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int. Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- Garcia-Vallejo J.J., van Liempt E., da Costa M.P. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through LewisY antigen expressed on ICAM-2. Mol. Immunol. 2008;45:2359–2369. doi: 10.1016/j.molimm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Kooyk Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS. 2003;111:698–714. doi: 10.1034/j.1600-0463.2003.11107803.x. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Torensma R., van Vliet S.J. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Kwon D.S., Torensma R. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Vliet S.J., van Duijnhoven G.C., Figdor C.G., van Kooyk Y. DC-SIGN, a dentritic cell-specific HIV-1 receptor present in placenta that infects T cells in trans – a review. Placenta. 2001;22(Suppl. A):S19–S23. doi: 10.1053/plac.2001.0674. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Engering A., van Kooyk Y. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J. Leukoc. Biol. 2002;71:921–931. [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Vliet S.J., Koppel E.A. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Vliet S.J., Engering A., ‘t Hart B.A., van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- Gringhuis S.I., den Dunnen J., Litjens M., van het Hof B., van Kooyk Y., Geijtenbeek T.B. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Guo Y., Feinberg H., Conroy E. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- Hauss P., Selz F., Cavazzana-Calvo M., Fischer A. Characteristics of antigen-independent and antigen-dependent interaction of dendritic cells with CD4+ T cells. Eur. J. Immunol. 1995;25:2285–2294. doi: 10.1002/eji.1830250826. [DOI] [PubMed] [Google Scholar]
- Hershkovitz O., Jarahian M., Zilka A. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology. 2008;18:28–41. doi: 10.1093/glycob/cwm125. [DOI] [PubMed] [Google Scholar]
- Hitchen P.G., Mullin N.P., Taylor M.E. Orientation of sugars bound to the principal C-type carbohydrate-recognition domain of the macrophage mannose receptor. Biochem. J. 1998;333:601–608. doi: 10.1042/bj3330601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmama Z., Sendide K., Talal A., Garcia R., Dobos K., Reiner N.E. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by a 1α,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J. Cell Sci. 2004;117:2131–2140. doi: 10.1242/jcs.01072. [DOI] [PubMed] [Google Scholar]
- Irjala H., Johansson E.L., Grenman R., Alanen K., Salmi M., Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J. Exp. Med. 2001;194:1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez D., Roda-Navarro P., Springer T.A., Casasnovas J.M. Contribution of N-linked glycans to the conformation and function of intercellular adhesion molecules (ICAMs) J. Biol. Chem. 2005;280:5854–5861. doi: 10.1074/jbc.M412104200. [DOI] [PubMed] [Google Scholar]
- Kessel J.M., Sedgwick J.B., Busse W.W. Ligation of intercellular adhesion molecule 3 induces apoptosis of human blood eosinophils and neutrophils. J. Allergy Clin. Immunol. 2006;118:831–836. doi: 10.1016/j.jaci.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Koppel E.A., Ludwig I.S., Hernandez M.S. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Koppel E.A., van Gisbergen K.P., Geijtenbeek T.B., van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- Kretz-Rommel A., Qin F., Dakappagari N. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J. Immunother. 2007;30:715–726. doi: 10.1097/CJI.0b013e318135472c. [DOI] [PubMed] [Google Scholar]
- Kwon D.S., Gregorio G., Bitton N., Hendrickson W.A., Littman D.R. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Lee B., Leslie G., Soilleux E. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 2001;75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekkerkerker A.N., van Kooyk Y., Geijtenbeek T.B. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr. HIV Res. 2006;4:169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- Liu H., Hwangbo Y., Holte S. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J. Infect. Dis. 2004;190:1055–1058. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- Liu W., Tang L., Zhang G. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- Ludwig I.S., Geijtenbeek T.B., van Kooyk Y. Two way communication between neutrophils and dendritic cells. Curr. Opin. Pharmacol. 2006;6:408–413. doi: 10.1016/j.coph.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Maeda N., Nigou J., Herrmann J.L. The cell surface receptor DC-SIGN discriminates between Myco-bacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- Mahnke K., Guo M., Lee S. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makola D., Peura D.A., Crowe S.E. Helicobacter pylori infection and related gastrointestinal diseases. J. Clin. Gastroenterol. 2007;41:548–558. doi: 10.1097/MCG.0b013e318030e3c3. [DOI] [PubMed] [Google Scholar]
- McGreal E.P., Miller J.L., Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megiovanni A.M., Sanchez F., Robledo-Sarmiento M., Morel C., Gluckman J.C., Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J. Leukoc. Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- Moraes T.J., Zurawska J.H., Downey G.P. Neutrophil granule contents in the pathogenesis of lung injury. Curr. Opin. Hematol. 2006;13:21–27. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- Moran A.P. Lipopolysaccharide in bacterial chronic infection: insights from Helicobacter pylori lipopolysaccharide and lipid A. Int. J. Med. Microbiol. 2007;297:307–319. doi: 10.1016/j.ijmm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Moran A.P. Relevance of fucosylation and Lewis antigen expression in the bacterial gastroduodenal pathogen Helicobacter pylori. Carbohydr. Res. 2008;343:1952–1965. doi: 10.1016/j.carres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Herre J., Brown G.D., Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J.E., Tedbury P.R., Homer-Vanniasinkam S., Walker J.H., Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Nigou J., Zelle-Rieser C., Gilleron M., Thurnher M., Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- Nortamo P., Li R., Renkonen R. The expression of human intercellular adhesion molecule-2 is refractory to inflammatory cytokines. Eur. J. Immunol. 1991;21:2629–2632. doi: 10.1002/eji.1830211049. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott E.M., O’Neill L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.G., Takahara K., Umemoto E. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Pastva A.M., Wright J.R., Williams K.L. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc. Am. Thorac. Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque S., Herrmann J.L., Duteyrat J.L. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem. J. 2005;392:615–624. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore A., Chatterjee A., Sood V. Risk for HIV-1 infection is not associated with repeat-region polymorphism in the DC-SIGN neck domain and novel genetic DC-SIGN variants among North Indians. Clin. Chim. Acta. 2008;391:1–5. doi: 10.1016/j.cca.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relloso M., Puig-Kroger A., Pello O.M. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- Robinson M.J., Sancho D., Slack E.C., LeibundGut-Landmann S., Reis e Sousa E. Myeloid C-type lectins in innate immunity. Nat. Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- Rohde K., Yates R.M., Purdy G.E., Russell D.G. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Russell D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- Sanabria-Valentin E., Colbert M.T., Blaser M.J. Role of futC slipped strand mispairing in Helicobacter pylori Lewis Y phase variation. Microbes Infect. 2007;9:1553–1560. doi: 10.1016/j.micinf.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Reiling N., Fessler C. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J. Immunol. 2008;180:6836–6845. doi: 10.4049/jimmunol.180.10.6836. [DOI] [PubMed] [Google Scholar]
- Schjetne K.W., Thompson K.M., Aarvak T., Fleckenstein B., Sollid L.M., Bogen B. A mouse C kappa-specific T cell clone indicates that DC-SIGN is an efficient target for antibody-mediated delivery of T cell epitopes for MHC class II presentation. Int. Immunol. 2002;14:1423–1430. doi: 10.1093/intimm/dxf110. [DOI] [PubMed] [Google Scholar]
- Serrano-Gomez D., Sierra-Filardi E., Martinez-Nunez R.T. Structural requirements for multimerization of the pathogen receptor DC-SIGN (CD209) on the cell surface. J. Biol. Chem. 2008;283:3889–3903. doi: 10.1074/jbc.M706004200. [DOI] [PubMed] [Google Scholar]
- Singer B.B., Klaile E., Scheffrahn I. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur. J. Immunol. 2005;35:1949–1959. doi: 10.1002/eji.200425691. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J. DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR):friend or foe? Clin. Sci. (London) 2003;104:437–446. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J., Coleman N. Langerhans cells and the cells of Langerhans cell histiocytosis do not express DC-SIGN. Blood. 2001;98:1987–1988. doi: 10.1182/blood.v98.6.1987. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J., Barten R., Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 2000;165:2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J., Morris L.S., Lee B. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J., Morris L.S., Leslie G. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- Starling G.C., McLellan A.D., Egner W. Intercellular adhesion molecule-3 is the predominant co-stimulatory ligand for leukocyte function antigen-1 on human blood dendritic cells. Eur. J. Immunol. 1995;25:2528–2532. doi: 10.1002/eji.1830250918. [DOI] [PubMed] [Google Scholar]
- Su S.V., Hong P., Baik S., Negrete O.A., Gurney K.B., Lee B. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J. Biol. Chem. 2004;279:19122–19132. doi: 10.1074/jbc.M400184200. [DOI] [PubMed] [Google Scholar]
- Tailleux L., Schwartz O., Herrmann J.L. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailleux L., Pham-Thi N., Bergeron-Lafaurie A. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Ip W.E., Michelow I.C., Ezekowitz R.A. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr. Opin. Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles J.B., Azad A.K., Schlesinger L.S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- van der Wel N., Hava D., Houben D. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- van Die I., van Vliet S.J., Nyame A.K. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- van Gisbergen K.P., Sanchez-Hernandez M., Geijtenbeek T.B., van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen K.P., Ludwig I.S., Geijtenbeek T.B., van Kooyk Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 2005;579:6159–6168. doi: 10.1016/j.febslet.2005.09.089. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., Geijtenbeek T.B. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., Appelmelk B., Geijtenbeek T.B. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 2003;9:153–159. doi: 10.1016/s1471-4914(03)00027-3. [DOI] [PubMed] [Google Scholar]
- van Liempt E., Imberty A., Bank C.M. Molecular basis of the differences in binding properties of the highly related C-type lectins DC-SIGN and L-SIGN to Lewis X trisaccharide and Schistosoma mansoni egg antigens. J. Biol. Chem. 2004;279:33161–33167. doi: 10.1074/jbc.M404988200. [DOI] [PubMed] [Google Scholar]
- van Liempt E., Bank C.M., Mehta P. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Vannberg F.O., Chapman S.J., Khor C.C. CD209 genetic polymorphism and tuberculosis disease. PLoS. ONE. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A., Ward C., Taylor E.L. Regulation of neutrophil apoptosis and removal of apoptotic cells. Curr. Drug Targets. Inflamm. Allergy. 2005;4:447–454. doi: 10.2174/1568010054526278. [DOI] [PubMed] [Google Scholar]
- Wang G., Ge Z., Rasko D.A., Taylor D.E. Lewis antigens in Helicobacter pylori: biosynthesis and phase variation. Mol. Microbiol. 2000;36:1187–1196. doi: 10.1046/j.1365-2958.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- Weis W.I., Drickamer K., Hendrickson W.A. Structureof a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Wu L., KewalRamani V.N. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liu Y.J. Development of dendritic-cell lineages. Immunity. 2007;26:741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Yan S.R., Sapru K., Issekutz A.C. The CD11/CD18 (β2) integrins modulate neutrophil caspase activation and survival following TNF-α or endotoxin induced transendothelial migration. Immunol. Cell Biol. 2004;82:435–446. doi: 10.1111/j.0818-9641.2004.01268.x. [DOI] [PubMed] [Google Scholar]


