Fig. 11.
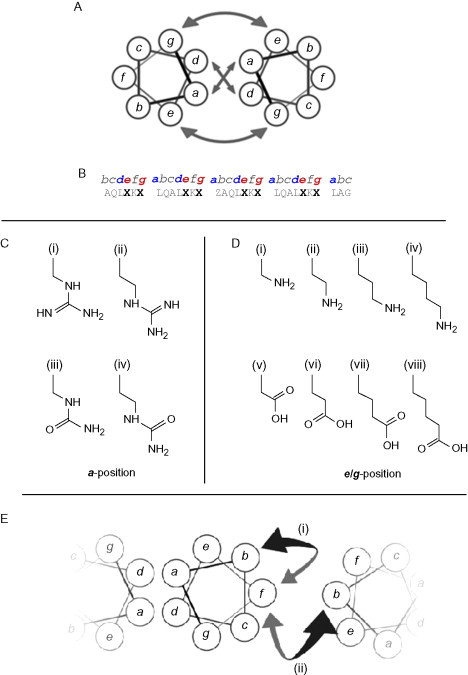
Coiled-coil designs that incorporate nonnative amino acids. (A) Helical wheel illustrating the main interactions available to designers of coiled-coil structures; the core and flanking salt-bridge interactions. (B) A “typical” base sequence for the investigation of nonnative amino-acid interactions in coiled coils.52., 53., 54. Residues in positions marked Z were substituted with amino acids with side chains as shown in C, while those marked X have been substituted with amino acids with side chains shown in D. (C) Guanidinium- and urea-based side chains with differing lengths, used to investigate core interactions. (i) guanidinylated diaminopropionic acid, (ii) guanidinylated diaminobutyric acid, (iii) pUr, a urea-terminated side chain, and (iv) pUr*, a urea-terminated side chain with an additional methylene group in the “linking” region to those in pUr. (D) Amino- and carboxyl-based side chains used to probe salt-bridge interactions. (i–iv) Positively charged, amine-terminated side chains with increasing length (and thus hydrophobic contact area): (iv) forms the naturally occurring lysine. (v–viii) Negatively charged, carboxyl-terminated side chains with increasing length. (vi) and (vii) form the natural amino-acids aspartic acid and glutamic acid, respectively. (E) Intra- and interhelical cation–π interactions investigated using the nonnative amino-acid norleucine.55
