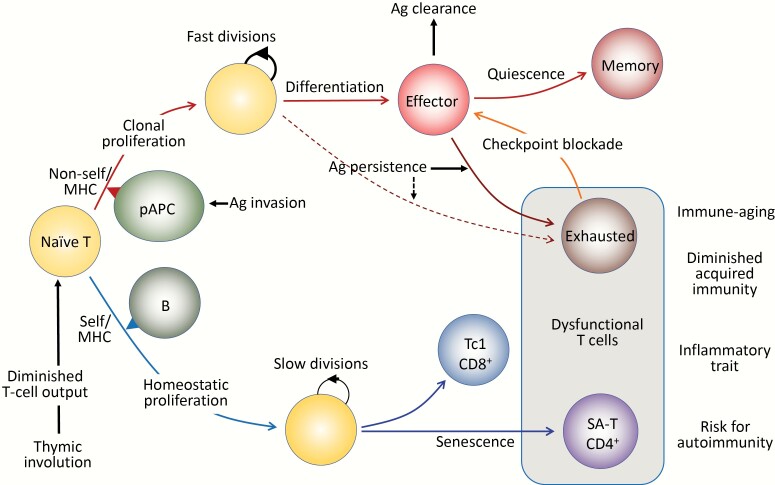
Fig. 1.
Antigen (Ag)-driven and antigen-independent generation of dysfunctional T cells. (Upper) In response to the optimal TCR stimulation via foreign antigens presented by professional antigen-presenting cells (pAPCs) expressing proper costimulatory molecules, specific naive T cells initiate robust clonal proliferation with fast cell divisions, followed by functional differentiation to various effector cells. As the antigens are cleared, the effector cells may die off, but a portion of them become quiescent and are maintained as central memory T cells. However, when antigen stimulation persists, the effector cells may go into a dysfunctional state via constitutive expression of checkpoint receptors such as PD-1 and LAG3 to prevent immunopathology due to excessive immune responses, called exhausted T cells. The exhausted T cells may also be derived from unique progenitor cells (pre-exhausted T cells). The function of exhausted T cells may be reverted with checkpoint blockade, although these T cells may eventually become refractory. (Lower) Naive T cells that developed through positive selection in the thymus have intrinsic affinity to self-MHCs and are under tonic TCR stimulation, mainly through B cells for CD4+ T cells. Although the tonic TCR signal per se is insufficient for causing proliferation, naive T cells initiate HP with slow cell divisions in the presence of sufficient amounts of homeostatic cytokines (IL-7, IL-15), which are increased in the lymphoid milieu in T-lymphopenic conditions. Thymic involution begins early in life with a progressive reduction of naive T-cell output over time, which increasingly drives HP of naive T cells with age to maintain the size of the T-cell pool in the periphery. Sustained HP of CD8+ T cells leads to the generation of CXCR3+ cells with increased capacity of IFNγ/TNFα production, predisposed to Tc1-type pro-inflammatory T cells. In CD4+ T cells, continuous HP results in the generation of dysfunctional T cells bearing the features resembling cell senescence, termed senescence-associated T (SA-T) cells. The SA-T cells are also characterized by the constitutive expression of PD-1 and LAG3, and partly CD153, although there is so far no evidence that the function is restored by PD-1 checkpoint blockade. SA-T cells are defective in proliferation and regular differentiation in response to optimal TCR stimulation, but these T cells secrete abundant pro-inflammatory cytokines, such as osteopontin and chemokines directed to innate inflammatory cells (Ccl3, 4), reminiscent of SASP. SA-T cells are progressively increased in proportion with age, but in addition, these T cells may remarkably accumulate in the GCs of lymphoid tissues or various other tissues under stresses or insults, predisposing to systemic autoimmunity or age-associated chronic inflammatory diseases.