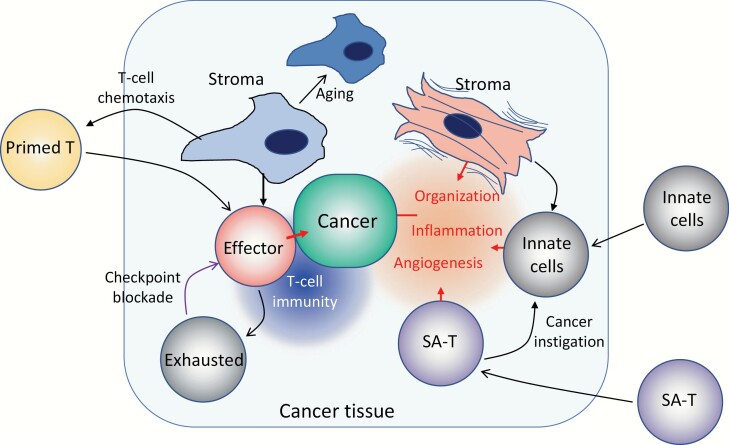
Fig. 3.
Schematic representation of T-cell dysfunction and tissue aging in cancer. In cancer tissues, various tissue reactions with often conflicting effects on cancer progression may occur, where tissue stroma cells play important roles. Certain stroma cell types may promote effective recruitment of primed T cells into the tissue via secretion of T-cell chemokines and forming a proper scaffold for their migration. Such T cells at a close enough vicinity to cancer cells proliferate and are activated to become effector cells destroying them. Sustained activation of the T cells, however, may lead to a dysfunctional state, or exhaustion, through checkpoint receptors, such as PD-1, the effects being pronounced when cancer cells express the ligands for checkpoint receptors. The process can be reverted with checkpoint-receptor blockade to resume effective T-cell immunity. The T-cell recruiting activity of stroma cells is radio-sensitive and is also diminished with age possibly through cellular aging. On the other hand, it is also known that stroma cells with potent pro-inflammatory activity are also increased in the tissues of certain types of cancers, often called myofibroblasts with contractile features. Such stroma cells recruit various innate immune cells to cause inflammatory reactions as well as neoangiogenesis and tissue organization. It is also likely that SA-T cells, which secrete abundant chemoattractants for inflammatory cells, such as osteopontin and Ccl3/4 as part of SASP, accumulate, exaggerating the formation of the inflammatory microenvironment around cancer cells. It is reported that osteopontin acts as a potent tumor-instigating factor recruiting myeloid cells directly from bone marrow. Overall, such an inflammatory microenvironment favors the progression of cancer, via damage to normal tissue integrity as well as suppression of T-cell immunity.