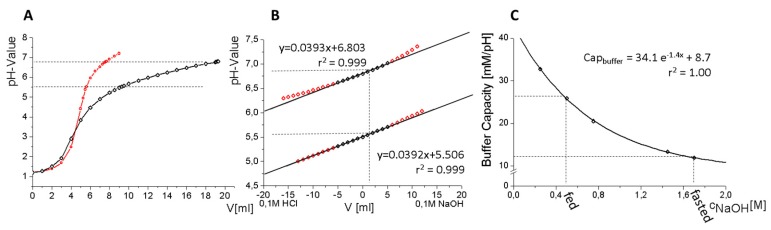
Figure 4.
(A) pH Profile of 50 mL 0.1 N HCl by adding potassium-citrate and tri-potassium-phosphate; red line represents different buffer capacities, and black line represents similar buffer capacities between pH 5.5–6.8. Buffer capacities are similar if the slope of the titration curves at pH = 5.5 and 6.8 are similar, which is the case for the black-colored curve; (B) titration profile of adjusted buffer at 5.5 and 6.8 to determine the buffer capacity at these levels. The adjusted buffers were titrated by using either 0.1 M NaOH or HCl. The slopes represent the buffer capacity at pH = 5.5 and pH = 6.8 (black line); (C) optimization of buffer capacity by adding sodium hydroxide to the concentrate to reach fasted and fed conditions at pH = 6.8.