Fig. 2.
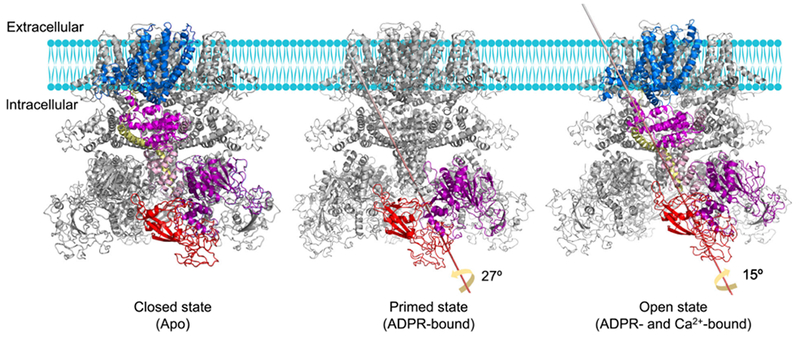
Molecular mechanism of human TRPM2 gating. In the apo state, NUDT9H mediates trans-interactions with the MHR1/2 domain, which may lock the channel in a closed conformation by limiting the rotation of the MHR arm. Upon ADPR engagement, MHR1/2 and NUDT9H undergo a large rotation, thereby disrupting the trans-interaction to prime the channel for opening. When both Ca2+ and ADPR bind, rotation of the cytosolic domains and movement of the TM region are integrated by TRP helix and then propagated to the S6 helix, leading to the enlargement of lower gate to increase the opening probability of the channel.
