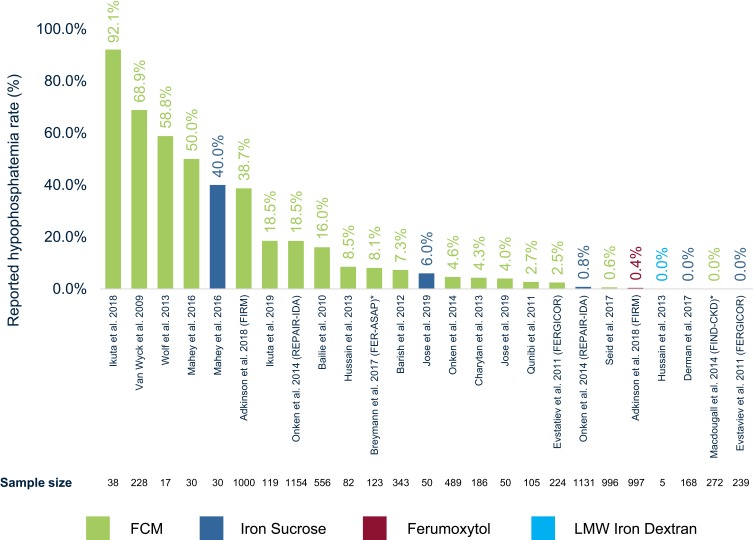
Figure 2.
Hypophosphatemia rate (%) reported per RCT.
Notes: Reported hypophosphatemia rates are given as noted in Table 1. Corresponding sample size of study treatment arm is shown below each study title. *Breymann et al31 report 0% hypophosphatemia treatment-emergent adverse events in patients; however, this study reports serum phosphate levels below the threshold of CTCAE grade 3 hypophosphatemia in 8.1% of patients. Macdougall et al33 report no “clinically significant hypophosphatemia”; however this study reports phosphate decrease in supplemental figures but without detailed information on number or % of patients falling below CTCAE threshold.
Abbreviations: RCT, randomized controlled trial; IVI, intravenous iron; FCM, ferric carboxymaltose; LMW, low-molecular-weight.