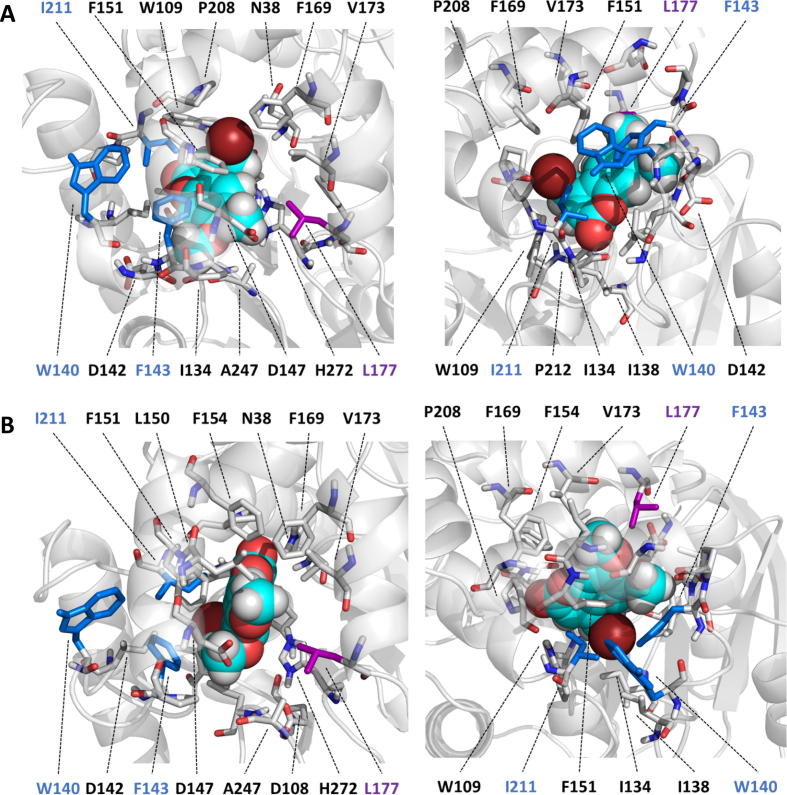
Fig. 4.
A) The NAC conformation of COU in LinBwt viewed from the main tunnel (left) and from the p3 tunnel (right). B) The tight binding conformation of COU in LinBwt viewed from the main tunnel (left) and from the p3 tunnel (right). In this binding mode, the carbonyl oxygen binds between the halide stabilizing residues N38 and W109. The cleaved halide (bromine) of COU is situated at the edge of the aromatic ring system which causes COU to mostly bind to p1 region in both binding modes. COU is shown as spheres, and residues within 4 Å from COU and the in LinB32 and LinB86 mutated residues are shown as sticks following color scheme of Fig. 1. COU is shown with the following color scheme: brown = chlorine, cyan = carbon, red = oxygen and white = hydrogen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)