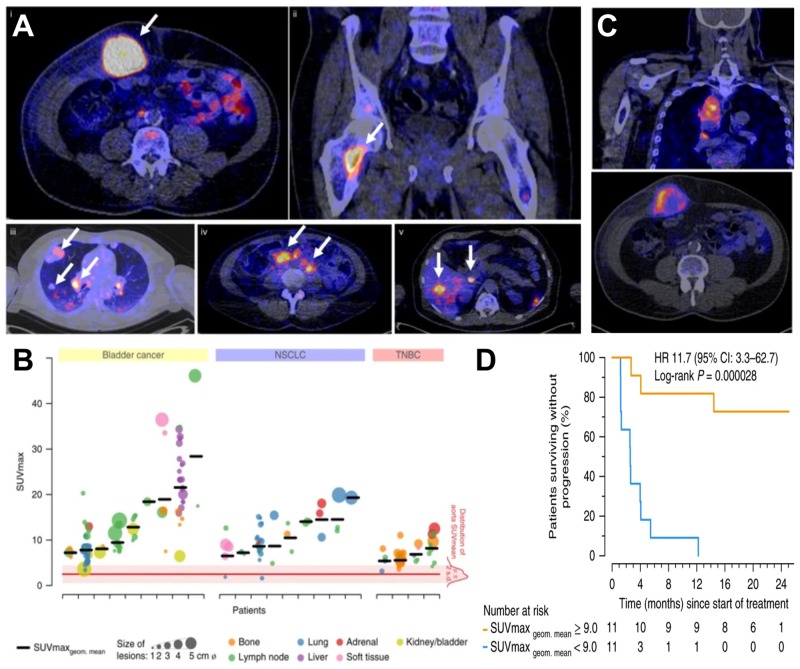
FIGURE 2.
Molecular imaging can be used as a non-invasive tool to predict clinical response of immunotherapy. (A) Examples of PET/CT images of four patients illustrating 89Zr-atezolizumab tumor uptake in five different locations on day 7 post-contrast agent administration (white arrows indicate tumor lesions; PET scans were performed once per patient and time point). Images (i) and (ii) are from the same patient, whereas images (iii), (iv), and (v) are each from a separate patient. (B) Overview of 89Zr-atezolizumab uptake as SUVmax at day 7 post-contrast agent administration in 196 tumor lesions with a diameter >2 cm grouped per tumor type and ordered by increasing geometric mean SUVmax per patient, visualizing tumor size and site, and with the distribution of aorta for blood pool background uptake as reference. Horizontal bars indicate geometric mean SUVmax per patient. (C) PET/CT images of lesions of two patients with heterogeneous intralesional 89Zr-atezolizumab uptake on day 7 post contrast agent administration. (Top) Mediastinal lesion of a NSCLC patient (SUVmax 19.9) and (Bottom) abdominal wall metastases of a bladder cancer patient (SUVmax 36.4). (D) Progression-free survival according to the geometric mean standard uptake value (SUVmax) per patient obtained by non-invasive PET imaging using 89Zr-labeled atezolizumab (orange, above-median geometric mean uptake; blue, below-median geometric mean uptake; N = 22 patients; two-sided log-rank test). For comparison, Hazard Ratios (HR) were only 2.6 and 1.3 for two different PD-L1 antibodies used in histology. For details see Bensch et al. (2018). (Reproduced with modifications from the indicated reference).