Abstract
A ten year-old Holstein cow had an intermittent bloody diarrhea, evolving to anorexia and recumbency, followed by death. Mycotic segmental enteritis was diagnosed based on the pathological and immunohistochemical findings. Rhizopus microsporus was identified as the causal agent through fungal culture and PCR analysis. Intestinal mucormycosis is poorly described in cattle and should, therefore, be included as a differential diagnosis in cases of diarrhea and death in ruminants, especially when there is disruption of the normal balance of the alimentary microbiota.
Keywords: Alimentary system, Mucormycosis, Mycotic, Diarrhea, Cattle
1. Introduction
Mucormycosis is a saprophytic opportunistic infection caused by fungi of the order Mucorales (former class Zygomycetes), of which several genera are able to cause disease in humans and animals [1,2], such as Rhizopus sp., Mucor sp., and Lichtheimia sp. (formerly Absidia sp. and Mycocladus sp.) [3]. Although zygomycosis has often been used as a synonym for mucormycosis, the former is currently considered more appropriate when fungal culture is available, while zygomycosis is a term that should be avoided [3].
Mucoralean fungi are ubiquitous in the environment, and may be found in vegetation, soil, and food [4,5]. Although Mucor sp., Rhizopus sp., and Lichtheimia sp. are residents of the ruminal microbiota, these fungi are able to cause systemic and deep fungal infections [6,7], which are often related to the immune status of the animal [[8], [9], [10]].
Mucormycosis affects commonly the forestomaches of cattle, mainly the rumen and omasum, following ruminal acidosis, mastitis, post-partum period, and/or prolonged use of antibiotics [1,8,11]. Mucoralean fungi may invade blood vessels and cause characteristic lesions, such as thrombosis, infarction, necrosis, and haemorrhage [8,10]. Although any segment of the gastrointestinal tract may be affected, intestinal involvement in cattle is rare [8]. This report describes the clinical, pathological, mycological, and molecular findings of segmental enteritis caused by Rhizopus microsporus in a cow.
2. Case
A ten year-old Holstein cow had a five-month history of intermittent diarrhea and weight loss (day of the first episode of diarrhea - day 0), with clinical improvement after antibiotic treatment and rumen transfaunation were performed. However, in the last seven days (day + 150), the cow presented new episodes of bloody diarrhea, evolving to anorexia at day +157 and recumbency at day +160, followed by death (day +161).
This cow was part of a herd composed by 48 dairy cattle in a property located at the city of Rolante in southern Brazil (29°39'03" S, 50°34'33" O). The herd was vaccinated annually for rabies, clostridiosis, and leptospirosis. Immediately after death, the cow was submitted for necropsy, and multiple samples of tissues were collected, fixed in 10% neutral buffered formalin, routinely processed for histology, and stained with hematoxylin and eosin (HE). Fresh samples of small intestine and mesenteric adipose tissue were also collected for further microbiological analysis.
At the necropsy, the cow was in poor body condition and presented pale mucous membranes. The small intestine had an extensive segmental mural thickening at the distal jejunum and proximal ileum with multiple petechiae and ecchymosis at the serosa (Fig. 1A). Within the lumen of this segment there was a firm, polypoid, irregular to multinodular greenish to yellowish mass, which partially obstructed the intestinal lumen, and measured approximately 3 cm in diameter (Fig. 1B). On the cut surface, the mass was predominantly solid and whitish intermixed by occasional friable areas, and the adjacent mesenteric adipose tissue was diffusely firm. No gross changes were observed within the forestomaches and within the mesenteric lymph nodes, as well as in other intestinal segments.
Fig. 1.
Gross and microscopic features of Rhizopus microsporus segmental enteritis in a cow. (A) Segmental mural thickening at the distal jejunum and proximal ileum with multifocal petechiae and ecchymosis involving the serosa. (B) The small intestine had a partially obstructive polypoid to multinodular, greenish to yellowish mass within the lumen. (C) Small intestine: the polypoid mass within the small intestine was characterized by marked fibrovascular tissue proliferation and a diffusely necrotic surface, which was covered by large amounts of fibrin intermixed by granular basophilic debris and marked inflammatory infiltrate (arrowheads). Within this area and extending into the submucosa there were multiple transverse and longitudinal sections of partially stained amphophilic hyphae (arrows), 200x, HE. (D) Small intestine: multiple broad thick-walled, non-septate, and branched hyphae with focal bulbous dilations were located within blood vessels, which also presented marked fibrinoid degeneration, 400x, Periodic acid-Schiff stain (PAS).
Microscopically, the small intestine mass was characterized by marked fibrovascular tissue proliferation at the base, with a diffusely ulcerated and necrotic surface, which was covered by large amounts of fibrin. Within this area and extending into the submucosa there were multiple transverse and longitudinal sections of partially stained broad amphophilic hyphae (Fig. 1C). These fungal structures often involved blood vessels, which presented thrombosis and fibrinoid degeneration. Moreover, a marked inflammatory infiltrate of neutrophils, lymphocytes, macrophages, and eosinophils surrounded these hyphae. The adjacent mesenteric adipose tissue showed multifocal to coalescent fibrinoid necrosis, which was intermixed by marked inflammatory infiltrate of neutrophils, in addition to moderate fibrinoid degeneration of blood vessels and thrombosis.
Sections of the small intestine mass and mesenteric adipose tissue were also stained with Grocott's methenamine silver (GMS) and periodic acid-Schiff (PAS). Both stains revealed multiple broad, thick-walled, non-septate, and branched hyphae in the small intestine mass section. These hyphae measured 6–8 μm in diameter and 10–70 μm in length, with focal bulbous dilations at the extremities (typical of zygomycete organisms) (Fig. 1D).
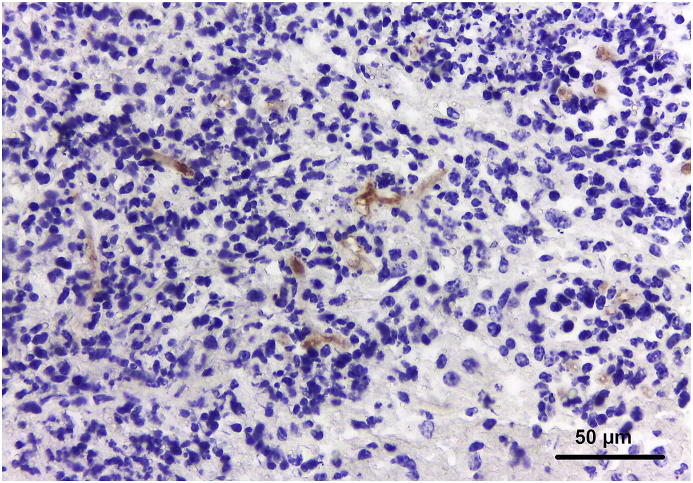
A section of the small intestine mass was submitted for immunohistochemistry (IHC) technique employing a monoclonal antibody anti-Rhizopus arrhizus (1:50 dilution, WSSA-RA-1, Bio-rad, Hercules, California, USA). The antigens were retrieved by boiling the sections for 40 min at 96 °C at a digital pressure cooker in Tris-EDTA buffer. The amplification signal was achieved by the peroxidase-labelled antibody method (MACH 4, Universal HRP-Polymer, Biocare Medical, Pacheco, California, USA). The reaction was revealed with 3,3’-diaminobenzidine (DAB, Sigma, St. Louis, Missouri, USA) chromogen, and the slide was counterstained with Mayer’s hematoxylin. Occasional fungal hyphae were highlighted in the submucosa with distinct labelling of the hyphal walls (Fig. 2).
Fig 2.
Small intestine: moderate immunostaining was observed at the fungal hyphae walls, 400x, IHC anti-Rhizopus arrhizus.
Tissue fragments (small intestine and mesenteric adipose tissue) were cultured onto Sabouraud Dextrose Agar plates, and, after 7 days of incubation at 25°-30 °C, fast growing and dark greyish-brown color with cottony aerial mycelia pure colonies were isolated. Microscopic examination showed that the colonies were composed of broad non-septate hyphae, sporangiospores, columella, and simple rhizoids. Based on the gross and micromorphology features, the isolate was consistent with members of the genus Rhizopus.
Additionally, DNA was extracted from these colonies using the Qiagen DNeasy® plant mini DNA extraction kit (Qiagen, Hilden, Germany) protocol, according to the manufacturer instructions. DNA extracted was detected with panfungal polymerase chain reaction (PCR) using ITS1-F and ITS4-R primers [12] for amplification of internal transcribed spacer 1 and 2 regions. This reaction was performed in a 25 μL mixture containing 1 μL of DNA extract, 12.5 μL Taq PCR master mix (Qiagen) and 0.5 μL of each primer (for a 0.2 μM final concentration of each primer). After a preincubation at 94 °C for 15 min, amplification was performed for a total of 35 cycles, as it follows: denaturation at 94 °C for 30s, annealing at 51 °C for 45s, extension at 72 °C for 1 min, and a final extension step of 10 min at 72 °C. PCR product was separated onto 2% agarose, purified using PureLink® PCR Purification Kit (Invitrogen, Carlsbad, California, USA), and sequenced to confirm the identity of the fungal isolate. Sequence alignment and comparison with sequences obtained from NCBI were done using the Geneious platform (Biomatters Ltd., Auckland, New Zealand). Sequencing analysis of the forward and reverse fragments revealed that the isolate was Rhizopus microsporus, with a similarity of 97 to 100% to sequences available at NCBI.
3. Discussion
Diagnosis of R. microsporus segmental enteritis was based on the clinical, pathological, and immunohistochemical findings, in addition to the identification of the fungus through mycological and molecular techniques. Diagnosis of mucormycosis is obtained either by fungal culture, IHC or histopathologic demonstration on tissue sections of non-septate (or rarely septate hyphae), broad and right-angled branching fungi [[13], [14], [15]]. IHC offers some advantages over culture including rapid results, preservation of tissue morphology allowing localization of the organism, and use of formalin-fixed stored paraffin-embedded tissues [16]. Nevertheless, the antibody anti-Rhizopus arrhizus may present cross-reactivity with other members of the family Mucoraceae [13], such as R. microsporus, which may restrain a further characterization at the genre/species level of the agent involved through this analysis [17]. Furthermore, fungal culture is relied as the main diagnostic method for the identification of Rhizopus sp.; nonetheless, it presents some limitations in regards to the identification at the species level [14]. Therefore, molecular detection through PCR technique has been recently employed and proved useful for the identification at the species level of Zygomycetes [14], as it was observed in this study, wherein R. microsporus was identified as the etiological agent related to segmental enteritis.
Mucoralean fungi pathogenesis in cattle relies on the fungal entrance through the gastrointestinal mucosae, which may result either in alimentary and/or systemic mycosis [18]. Fungal infections are prone to occur when there is disruption of the normal balance of the alimentary microbiota, which has been associated with several factors, such as ruminal acidosis, broad-spectrum antibiotics treatment [1,5,8], and stress at the post-partum period in cows [5,8]. Therefore, in the present study, it is suggested that multiple predisposing factors, such as prolonged use of antibiotics and stress related to the post-partum period, may have favored the passage of the fungus to the lower digestive tract, with subsequent fungal invasion of the intestinal mucosa.
Although rare in cattle, mucormycosis is a relatively common condition in humans, in which it is mainly associated with immunodeficiency disorders, such as Crohn's disease, HIV infection, and malnutrition. Affected organs include the stomach, small and large intestines with ulcerative and hemorrhagic lesions, often associated with perforations, or proliferative lesions mimicking tumors [4,9,10,19,20]. Moreover, mucormycosis in cattle usually involves the anterior portions of the digestive tract (rumen and omasum) with necrotic lesions [8,11], while in the present case the lesion was restricted to the jejunum-ileum with a proliferative lesion.
The fungal characteristics observed through histopathology, histochemical techniques, immunohistochemistry, and fungal culture associated with PCR technique confirmed that R. microsporus was the etiological agent of these lesions. This latter exam allowed us to obtain a consistent characterization of the fungal species involved, and, thus, to determine the condition as mucormycosis. Intestinal mucormycosis is still poorly described in cattle, and R. microsporus segmental enteritis should be included as a differential diagnosis in cases of diarrhea and death in ruminants, especially when some predisposing factors, such as prolonged use of antibiotics and stress related to the post-partum period, are present.
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Consent
Written informed consent was obtained from the patient or legal guardian(s) for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for supporting this study.
References
- 1.Stefaniak T., Houszka M., Nowaczyk R., Rouibah K., Jawor P. Zygomycosis of the abomasum in neonatal calves during treatment of diarrhea caused by Escherichia coli: a case report. Med. Weter. 2016;72:263–267. [Google Scholar]
- 2.Seyedmousavi S., Bosco S., De Hoog S., Ebel F., Elad D., Gomes R.R., Jacobsen I.D., Martel A., Mignon B., Pasmans F., Piecková E., Rodrigues A.M., Singh K., Vicente V.A., Wibbelt G., Wiederhold N.P., Guillot J. Fungal infections in animals: a patchwork of different situations. Med. Mycol. 2018;56:S165–S187. doi: 10.1093/mmy/myx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon-Chung K.J. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin. Infect. Dis. 2012;54 doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng V.C.C., Chan J.F.W., Ngan A.H.Y., To K.K.W., Leung S.Y., Tsoi H.W., Yam W.C., Tai J.W.M., Wong S.S.Y., Tse H., Li I.W.S., Lau S.K.P., Woo P.C.Y., Leung A.Y.H., Lie A.K.W., Liang R.H.S., Que T.L., Ho P.L., Yuen K.Y. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 2009;47:2834–2843. doi: 10.1128/JCM.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega J., Uzal F.A., Walker R., Kinde H., Diab S.S., Shahriar F., Pamma R., Eigenheer A., Read D.H. Zygomycotic lymphadenitis in slaughtered feedlot cattle. Vet. Pathol. 2010;47:108–115. doi: 10.1177/0300985809352975. [DOI] [PubMed] [Google Scholar]
- 6.Lund A. Yeasts and moulds in the bovine rumen. J. Gen. Microbiol. 1974;81:453–462. doi: 10.1099/00221287-81-2-453. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura M., Toyota Y., Ishida Y., Nakaya H., Kameyama K., Nishikawa Y., Miyahara K., Inokuma H., Furuoka H. Zygomycotic mediastinal lymphadenitis in beef cattle with ruminal tympany. J. Vet. Med. Sci. 2014;76:123–127. doi: 10.1292/jvms.13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen H.E., Olsen S.N., Aalbæk B. Gastrointestinal aspergillosis and zygomycosis of cattle. Vet. Pathol. 1994;31:28–36. doi: 10.1177/030098589403100104. [DOI] [PubMed] [Google Scholar]
- 9.Morton J., Nguyen V., Ali T. Mucormycosis of the intestine: a rare complication in Crohn's disease. Gastroenterol. Hepatol. 2012;8:137–140. [PMC free article] [PubMed] [Google Scholar]
- 10.Choi W.T., Chang T.T., Gill R.M. Gastrointestinal zygomycosis masquerading as acute appendicitis. Case Rep. Gastroenterol. 2016;10:81–87. doi: 10.1159/000444275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda T., Tabuchi K., Shirota K., Une Y., Nomura Y. Mucormycosis in a cow. Nippon Juigaku Zasshi. Japanese J. Vet. Sci. 1987;49:527–530. doi: 10.1292/jvms1939.49.527. [DOI] [PubMed] [Google Scholar]
- 12.White Tj T.J., Bruns T., Lee S. Amplication and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: MA Innis T.W., Gelfand D.H., Sninsky J.J., editors. PCR Protoc. A Guid. to Methods Appl. Academic P; San Diego: 1990. pp. 315–322. [Google Scholar]
- 13.Jensen H.E., Aalbæk B., Lind P., Krogh H.V. Immunohistochemical diagnosis of systemic bovine zygomycosis by murine monoclonal antibodies. Vet. Pathol. 1996;33:176–183. doi: 10.1177/030098589603300207. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz P., Gantier J., Garcia-hermoso D., Lortholary O., Dannaoui E., Mycologie L.D.P. Molecular identification of zygomycetes from culture and experimentally infected tissues. J. Clin. Microbiol. 2006;44:340–349. doi: 10.1128/JCM.44.2.340-349.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman C.A., Malani A.N. Zygomycosis: an emerging fungal infection with new options for management. Curr. Infect. Dis. Rep. 2007;9:435–440. doi: 10.1007/s11908-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 16.Schuetz A.N., Cohen C. Aspergillus immunohistochemistry of culture-proven fungal tissue isolates shows high cross-reactivity. Appl. Immunohistochem. Mol. Morphol. 2009;17:1–6. doi: 10.1097/PAI.0b013e3181a38e05. [DOI] [PubMed] [Google Scholar]
- 17.Jung J., Park Y.S., Sung H., Song J.S., Lee S.O., Choi S.H., Kim Y.S., Woo J.H., Kim S.H. Using immunohistochemistry to assess the accuracy of histomorphologic diagnosis of aspergillosis and mucormycosis. Clin. Infect. Dis. 2015;61:1664–1670. doi: 10.1093/cid/civ660. [DOI] [PubMed] [Google Scholar]
- 18.Angus K.W., Gilmour N.J.L., Dawson C.O. Alimentary mycotic lesions in cattle: a histological and cultural study. J. Med. Microbiol. 1973;6:207–213. doi: 10.1099/00222615-6-2-207. [DOI] [PubMed] [Google Scholar]
- 19.Brad Spellberg A.I., Edwards John., Jr. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekkers R., Verweij P., Weemaes C., Severijnen R., Van Krieken J., Warris A. Gastrointestinal zygomycosis due to Rhizopus microsporus var. rhizopodiformis as a manifestation of chronic granulomatous disease. Med. Mycol. 2008;46:491–494. doi: 10.1080/13693780801946577. [DOI] [PubMed] [Google Scholar]