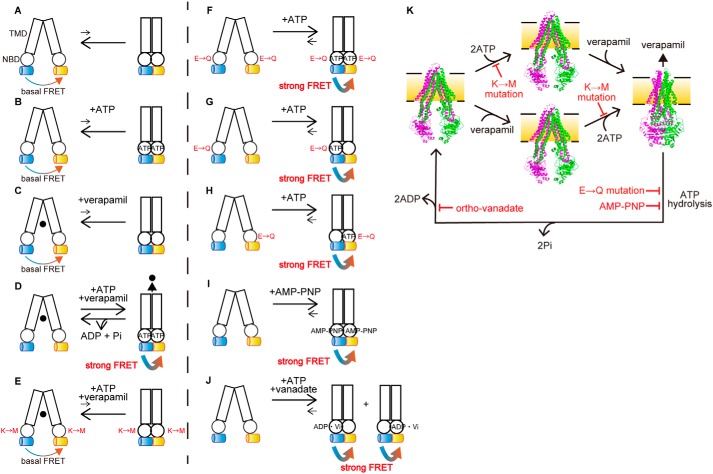
Figure 8.
Schematic illustration of the ATP-dependent conformational changes of P-gp. A–K, transition equilibria between the inward-facing conformation (left), which generates basal FRET, and the outward-facing conformation (right), which generates strong FRET, of human P-gp are indicated by arrows. Rectangles and circles represent TMDs and NBDs, respectively. Blue and yellow cylinders represent mCerulean and mVenus, respectively. The replacement of Walker A lysine by methionine and replacement of catalytic glutamate by glutamine are shown as M and Q, respectively. ●, verapamil. The inward-facing conformation is stable in the absence of ATP or verapamil (A–C). The presence of ATP and verapamil causes the transition from the inward-facing to the outward-facing conformation and generates strong FRET (D). Replacement of the Walker A lysine by methionine prevents ATP binding and generates basal FRET (E). Replacement of the Walker B glutamate by glutamine in either NBD stabilizes the outward-facing state and generates strong FRET (F–H). Binding of MgAMP-PNP stabilizes the outward-facing state and generates strong FRET (I). Orthovanadate traps P-gp in the stable post-ATP hydrolysis transition state (probably in either NBD) to generate strong FRET (J). K, model of the transport cycle of P-gp caused by ATP. The inward-facing conformation is stable in the absence of ATP or verapamil (left and middle). The presence of ATP and verapamil causes the transition from the inward-facing to the outward-facing conformation (right). ATP hydrolysis and subsequent release of γ-phosphate from both NBDs allow P-gp to return to the inward-facing state. Mutations of the Walker A lysine prevents ATP binding. Mutations of the Walker B glutamate or binding of AMP-PNP inhibits ATP hydrolysis. Orthovanadate traps P-gp in the stable post-ATP hydrolysis transition state. The inward-facing conformation of mouse P-gp is shown in PDB 4m1m; the outward-facing conformation of human P-gp is shown in PDB 6c0v.