Figure 10.
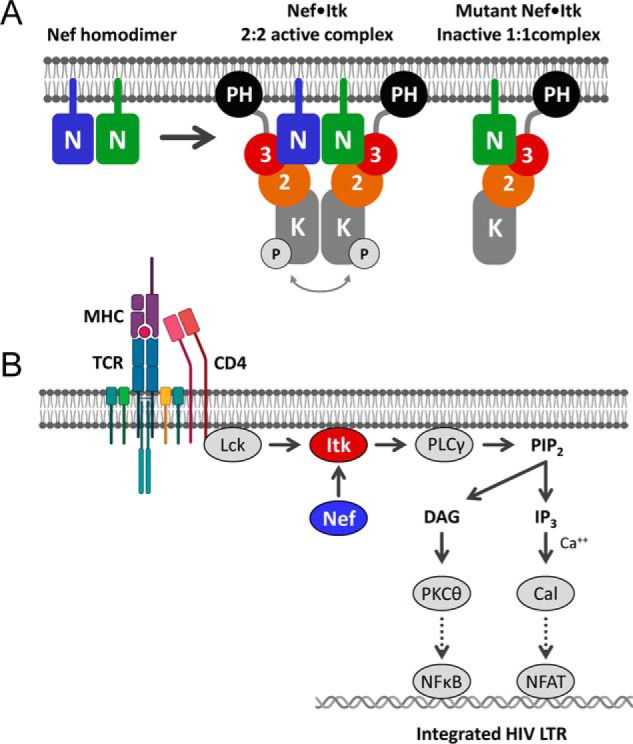
Models of Nef-mediated Itk activation and consequences for HIV-1 transcription. A, Nef forms homodimers at the cytoplasmic face of the plasma membrane, which stabilize Itk homodimers as a 2:2 complex. Regulatory domain displacement and kinase domain juxtaposition contribute to sustained kinase activation via autophosphorylation in trans. Dimerization-defective Nef mutants retain interaction with Itk at the membrane but form inactive 1:1 complexes. N, Nef; 3, Src homology 3 domain; 2, Src homology 2 domain; K, kinase domain; P, activation loop phosphorylation. B, proposed consequences of constitutive Itk activation by Nef for HIV-1 transcription in CD4 T cells. The T-cell receptor (TCR) complex is normally activated by antigen-loaded MHC molecules. The TCR activates the T cell–specific Src-family kinase Lck, which is associated with the cytosolic tail of the co-receptor, CD4. Active Lck directly phosphorylates Itk on its activation loop, which in turn phosphorylates and activates phospholipase Cγ (PLCγ). Active PLCγ generates the second messenger diacylglycerol (DAG) and inositol triphosphate (IP3) via hydrolysis of membrane phosphatidylinositol 4,5-bisphosphate (PIP2), ultimately leading to activation of protein kinase Cθ (PKCθ) and the calcium-dependent protein serine/threonine phosphatase, calcineurin (Cal), respectively. Protein kinase Cθ promotes activation of NF-κB via the CARMA1/BCL10/MALT1 complex (not shown), whereas calcineurin dephosphorylates NFAT to drive nuclear localization. Both NF-κB and NFAT participate in transcription of the integrated HIV-1 provirus. The data presented here support direct activation of Itk by Nef at the membrane downstream of the TCR. Additional details of TCR signaling are omitted for clarity. The cartoon was adapted from Gaud et al. (46). LTR, long terminal repeat.
