Figure 1.
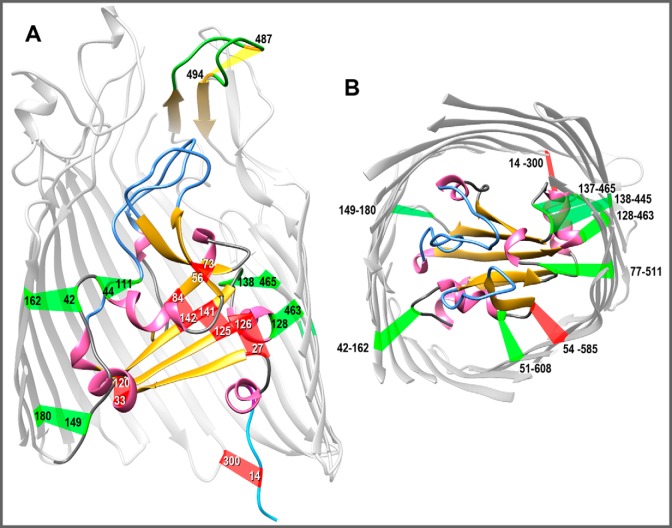
Native and engineered Cys pairs in FepA. From the crystal structure of FepA (1FEP (8)), we modeled the polypeptide (using the Modeller function of CHIMERA) to contain 15 novel Cys pairs, in addition to the native Cys-487–Cys-494 disulfide in L7 of FepA that is resistant to modification unless reduced (46). A, side view of Cys pairs. The structure of FepA encompasses a 150-residue globular N-domain within a 574-residue C-terminal β-barrel (only partially rendered to allow a better view of the N-domain). With few exceptions, FepA orthologs contain a natural Cys pair (residues 487–494 in E. coli FepA) in L7 (yellow). The flexibility of L7 prevented its delineation in 1FEP (8), but Modeller predicted its disposition. We engineered six individual Cys pairs within the internal globular domain: 27–126, 33–120, 44–111, 56–73, 84–142, and 125–141. We also constructed nine Cys pairs that conjoin the surface of the N-domain and the interior of the β-barrel: 14–300 (37), 42–162, 51–608, 54–585, 77–511, 128–463, 137–465, 138–445, and 149–180. B, top view of Cys pairs. We hid the surface loops in the model shown in A and rotated it 90° on the x axis to reveal the interior of the channel. In both panels, α-helices are pink; β-strands are gold; N-domain loops are blue; and putative disulfide bonds formed by the Cys pairs are colored red if they inhibited FeEnt uptake or green if they did not inhibit FeEnt uptake, as described in the text. The images were rendered from PDB code 1FEP (8) by CHIMERA (UCSF).