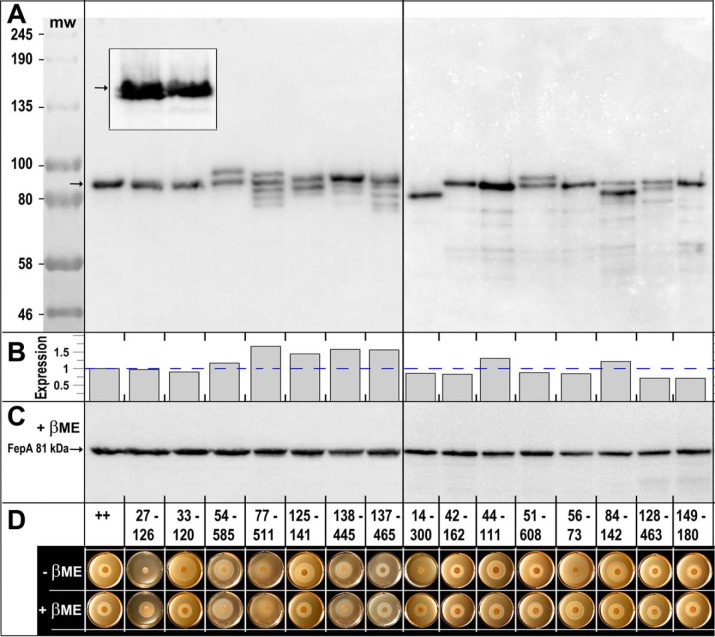
Figure 2.
A, SDS-PAGE/western immunoblot analysis of disulfide formation by engineered Cys pairs. After growth of E. coli OKN3 harboring fepA mutants on the low-copy plasmid pHSG575 (75), in iron-deficient MOPS minimal media, we prepared cell-envelope fragments from E. coli OKN3 expressing WT FepA and each of the 15 Cys-pair mutants. We subjected the samples to SDS-PAGE (in the absence of any reducing agent), transferred the proteins to nitrocellulose, and performed western immunoblots with anti-FepA MAbs 41 and 45 (43), developed with 125I-protein A (33). Five of the mutant proteins (33–120, 42–162, 44–111, 56–73, and 149–180) had the same electrophoretic mobility as native FepA: a single band at 81.5 kDa (76). Conversely, 10 of the mutant proteins showed single (14–300) or multiple (27–126, 54–585, 77–511, 125–141, 51–608, 84–142, 128–463, 137–465, and 138–445) immunoreactive bands with altered mobility, suggesting that they were fully or partially disulfide-bonded. The illustration derives from two separate immunoblots, separated by a vertical line. The gels also contained pre-stained molecular weight markers (New England Biolabs, catalog no. P77195) that we photographed on the nitrocellulose and added to the immunoblot images. The inset shows a magnified view, from another blot, of mutants 27–126 and 33–120. The former Cys pair forms two immunoreactive bands of nearly indistinguishable electrophoretic mobility, indicating that this Cys pair does form a disulfide bond. 33–120, conversely, migrates as a single band. B, quantification of FepA expression. The arrows in A, C, and the inset define the position of WT FepA that migrates at ∼81 kDa (76). The immunoblot in A was developed with 125I-protein A and scanned on a Typhoon Biomolecular imager, which allowed quantification of the immunoreactive material in each lane, using ImageJ (73). We normalized the expression values to the level of WT FepA (dashed blue line): Cys-pair mutant expression ranged from 0.71 to 1.67 of WT FepA expression; the mean expression among 15 mutants was 0.95 of WT FepA. C, we subjected the same samples as in A to reduction by 3 mm βME, resolved them on a duplicate SDS-polyacrylamide gel, transferred the proteins to NC, and immunoblotted as described in A. This immunoblot, as well as that of A, contains WT FepA, that provides an internal molecular mass marker at 81.5 kDa. D, FeEnt nutrition tests in the absence and presence of βME. We evaluated the ability of the WT FepA (++) and its Cys-pair mutants to acquire FeEnt in nonreducing or reducing conditions.