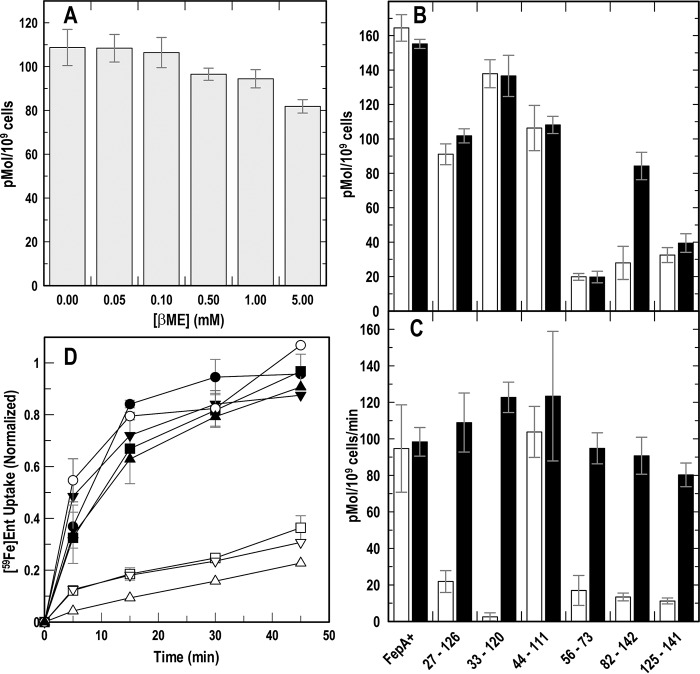
Figure 3.
[59Fe]Ent binding and transport by intra-N Cys-pair mutants of FepA. After growth in iron-deficient MOPS medium, we tested E. coli expressing WT FepA or its Cys-pair mutants for binding and transport of [59Fe]Ent (74). A, effect of βME on [59Fe]Ent binding to WT FepA. The FeEnt binding capacity of OKN3 (ΔfepA)/pITS23 (fepA+) slightly decreased (up to 20%) in the presence of 5 mm βME. B, [59Fe]Ent binding to Cys-pair mutants: binding capacities in the presence of saturating (10 nm) [59Fe]Ent, in the absence or presence of 5 mm βME. C, [59Fe]Ent uptake by Cys-pair mutants: Vmax in the presence of saturating (10 nm) [59Fe]Ent, in the absence or presence of 5 mm βME. D, accumulation of [59Fe]Ent: rates of [59Fe]Ent uptake in the absence (open symbols) and presence (filled symbols) of 5 mm βME by WT FepA (○), 27–126 (□), 33–120 (▵), and 125–141 (▿). βME restored WT FeEnt uptake to all the intra-N Cys-pair mutants.