Short abstract
Objective
Overexpression of microRNA-21 (miR-21) increases the radiation resistance of esophageal squamous cell carcinoma (ESCC). However, the molecular mechanism responsible for this action is still unclear. In the present study, we investigated the role of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in miR-21-enhanced radiation resistance in patients with ESCC.
Methods
We evaluated the association between miR-21 levels and radiation resistance in patients with ESCC. We also investigated the role of PTEN in the proliferation and apoptosis of ESCC cells transfected with miR-21 inhibitor during irradiation, using PTEN small interfering RNA (siRNA).
Results
MiR-21 levels were significantly higher in radiation-resistant patients. Downregulation of miR-21 during irradiation suppressed the radiation resistance of ESCC cells, demonstrated by decreased cell proliferation and increased cell apoptosis. PTEN siRNA attenuated miR-21-induced suppression of radiation resistance in ESCC cells.
Conclusions
These results suggest that miR-21 enhanced the radiation resistance of ESCC by inhibiting PTEN. MiR-21 and PTEN are potential therapeutic biotargets for ESCC.
Keywords: miR-21, PTEN, radiation resistance, esophageal squamous cell carcinoma, cell proliferation, apoptosis
Introduction
Esophageal cancer is a relatively common high-risk tumor worldwide and a major cause of cancer-related death. About half of all cases of esophageal cancer occur in China.1 Esophageal cancer can be divided pathologically into squamous cell carcinoma and adenocarcinoma, with squamous cell carcinoma mainly prevalent in developing countries and adenocarcinoma more common in developed countries, while other types are rare. Most cases in China are esophageal squamous cell carcinoma (ESCC), which accounts for around 90% of all cases in this country.2 The survival rate of patients with ESCC is less than 10%,3 and the 5-year overall survival rate is 20% to 40%.4 Surgery, radiotherapy, and chemotherapy are the three main routine treatments for esophageal cancer. However, owing to the physiological anatomy of the esophagus, the atypical early symptoms, and a lack of effective early-diagnosis methods, most patients with esophageal cancer are diagnosed at an advanced stage and are therefore unsuitable for radical surgery.5 Radiotherapy is thus the main treatment method for advanced esophageal cancer, and more than half of all patients receive radiation during their treatment. However, surviving tumor cells may develop radiotolerance, resulting in tumor recurrence after the radiosensitive cells are killed by radiation therapy.6
Activation of oncogenes and inactivation of tumor suppressor genes leading to abnormalities in intercellular signaling pathways may be one mechanism leading to radiation resistance in tumor cells.7 Numerous recent studies have shown that micro RNAs (miRNAs) are involved in many cellular regulatory processes, including activation of different signaling pathways and induction of apoptosis.8–10 Furthermore, miRNA expression is involved in the regulation of radiation resistance in tumor cells.11,12 MiRNAs are a group of small, conservative, non-coding RNAs that degrade or repress their translation target mRNAs by direct binding to the 3′-untranslated region.13 MiR-21 is overexpressed in many solid tumors.14 Meng et al. reported that phosphate and tension homology deleted on chromosome 10 (PTEN) was a target gene of miR-21,15 and miR-21 inhibited PTEN and promoted the growth and invasion of non-small cell lung cancer and pancreatic cancer cells.16,17 Liu et al. reported that miR-21 promoted the growth, metastasis, and chemoresistance or radioresistance of non-small cell lung cancer cells by targeting PTEN,18 and subsequent studies confirmed that miR-21 could regulate the radioresistance of tumor cells through PTEN.19,20 Li et al.21 also reported that abnormal regulation of miR-21 promoted the progression of esophageal cancer.
Based on the above studies, we hypothesized that miR-21 might enhance the radioresistance of tumor cells by inhibiting the expression of PTEN in ESCC. We therefore investigated the involvement of miR-21 in mediating radioresistance in ESCC. We also examined the role of PTEN as a target gene of miR-21 in the regulation of radiosensitivity, and considered the potential of miR-21 as a therapeutic target for radiosensitization in patients ESCC.
Materials and methods
Patients and specimens
Tumor specimens were obtained from 40 consecutive patients with ESCC admitted to Hebei Medical University between September 2015 and December 2017. All patients received radiotherapy and were divided into a radiation-resistant group (20 patients with <40% reduction of tumor size after radiation treatment) and a radiation-sensitive group (20 patients with >60% reduction of tumor size after radiation treatment). The tissue specimens were stored at −193°C until use. The study was approved by the Ethics Research Committee of Fourth Hospital, Hebei Medical University (HU406AF). Signed informed consent, in accordance with the Declaration of Helsinki, was obtained for each donated tumor sample.
Cell culture and ionizing radiation
The human ESCC TE-1 cell line was purchased from the Chinese Academy of Sciences (Shanghai, China). The cells were grown in RPMI-1640 medium with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in 5% CO2. Cells in logarithmic growth phase were used for all experiments. Cells were exposed to 0, 2, 4, 6, and 8 Gy irradiation, respectively, using a Model 143 137cesium γ-irradiator (JL Shepherd & Associates, San Fernando, CA, USA) at a rate of 2.4 Gy/minute.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using an Absolutely RNA™ RT-PCR Miniprep Kit (Stratagene, China), in accordance with the manufacturer’s instructions. Reverse transcription was carried out using 10 ng of RNA in a volume of 5 µL, with 3 µL of primer. RT-PCR was performed using the TaqMan probe method (PrimeScript RT reagent Kit, TaKaRa Biotechnology, Dalian, China), in accordance with the manufacturer’s instructions. PCR was performed with an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers used in this study were as follows: miR-21-5p: UAGCUUAUCAGACUGAUGUUGA, miR-21-5p R: CTCAACTGGTGTCGTGGAGTC GGCAATTCAGTTGAGTCAACATC, miR-21-5p F: ACACTCCAGCTGGGTAGCTTATCAG ACTGATG, all R: CTCAACTGGTGTCGTGGA, U6F: CTCGCTTCGGCAGCACA, U6R: AACGCTTCACGAATTTGCGT. U6 was used for standardization and relative expression levels were calculated using the 2-ΔΔCt method.
Cell transfection
We constructed an miR-21 inhibitor vector and PTEN small interfering RNA (siRNA). A negative control (NC) group was treated with a scrambled sequence with no significant homology to any rat, mouse, or human gene. The cells were transfected using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer's instructions. MiR-21 expression was assessed by qRT-PCR at 48 hours after transfection.
Cell proliferation assay
We evaluated the effect of miR-21 expression on cell proliferation 48 hours after transfection using a Cell Counting Kit-8 (CCK-8, Beyotime, China). The radioresistance of the transfected cells was assessed by plating in 96-well plates and exposure to 8 Gy γ-radiation. Cells were removed at 1, 2, 3, and 4 days, CCK-8 (10 µL) was added, and the cells were then incubated at 37°C for 1.5 hours. Finally, cell viability was measured at 450 nm using a microplate reader.
Cell apoptosis
Apoptosis was detected by annexin V-fluorescein isothiocyanate (FITC) staining. The cells were irradiated for 8 hours at 8 Gy and then collected. The supernatant was removed and the cell monolayer was gently washed with phosphate-buffered saline (PBS). The cells were then digested with trypsin, digestion was terminated with PBS containing 10% serum, and the cells were resuspended in ice-cold PBS, fixed with 70% ethanol, and stained using an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA), in accordance with the manufacturer's instructions. Fluorescence was measured using a flow cytometer (BD Biosciences) and the rate of apoptosis was analyzed.
Western blot
Protein expression was detected by western blot. Total protein was extracted by conventional radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China), in accordance with the manufacturer’s instructions. The extracted protein was then quantified by the bicinchoninic acid assay (Cell Signaling Technology, Danvers, MA, USA), in accordance with themanufacturer’s instructions. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroporated to polyvinylidene difluoride membranes, blocked in 5% skim milk for 1.5 hours, and then incubated with primary antibody on a shaker at 4°C overnight. The primary antibodies used were anti-PTEN (1:200, Abcam, Cambridge, MA, USA) and anti-cleaved-poly (ADP-ribose) polymerase (PARP), and anti-cleaved-caspase 3 (1:1000; Cell Signaling Technology). The blots were then incubated with horseradish peroxidase-conjugated secondary PARP antibody (1:100; Cell Signaling Technology) for 1 hour. Glyceraldehyde 3-phosphate dehydrogenase antibody was used as a control. Finally, the blots were visualized by enhanced chemiluminescence with horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody (Cell Signaling Technology) for 1 hour at room temperature.
Statistical analyses
The data were presented as mean ± standard deviation. All statistical analyses were performed using SPSS for Windows, Version 15.0 (SPSS Inc., Chicago, IL, USA) in at least three independent experiments. Two-tailed Student’s t-tests or one-way ANOVA were used to analyze the differences among the groups. P<0.05 was considered significant.
Results
MiR-21 levels were increased in radiation-resistant compared with radiation-sensitive patients
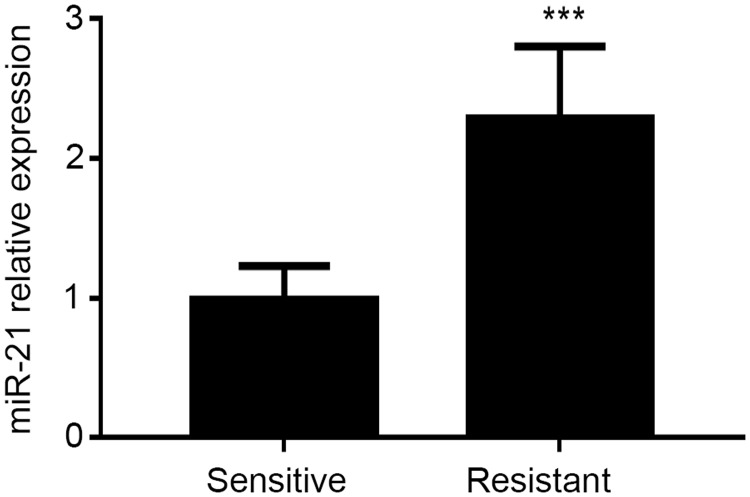
MiR-21 was overexpressed in ESCC, and downregulation of miR-21 suppressed the radiation resistance of ESCC.22 We detected miR-21 expression levels in 20 radiation-resistant and 20 radiation-sensitive ESCC patients using qRT-PCR. MiR-21 levels were significantly higher in the radiation-resistant patients (P<0.05) (Figure 1), confirming that high miR-21 levels were significantly correlated with radiation resistance in ESCC.
Figure 1.
Expression of miR-21 in radiation-resistant and radiation-sensitive esophageal squamous cell carcinoma, detected by qRT-PCR. ***P<0.001.
MiR-21 was associated with radiation resistance in ESCC cells
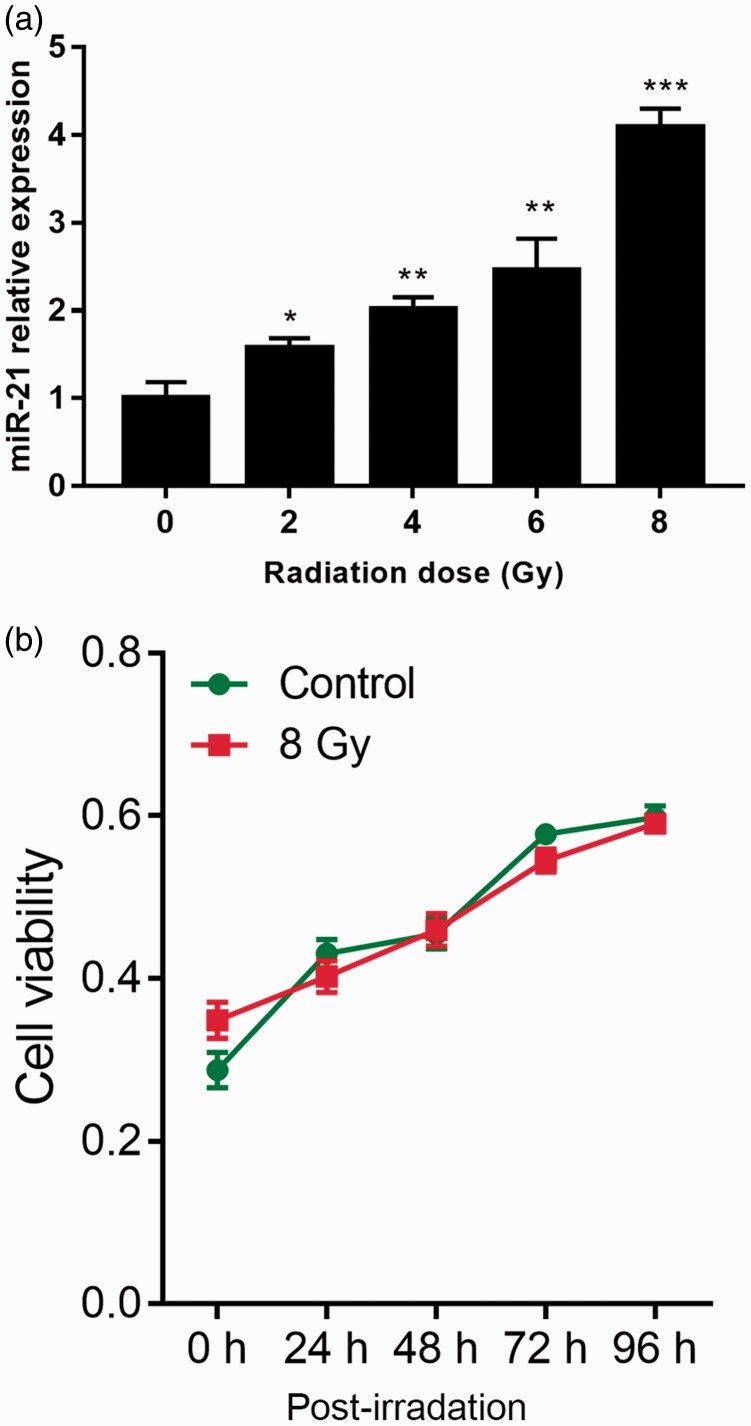
MiR-21 expression was examined in TE-1 cells after radiation with 0, 2, 4, 6, and 8 Gy. MiR-21 expression was increased by radiation in a dose-dependent manner in these cells (Figure 2a), indicating that miR-21 was associated with radiation in ESCC cells. The viability of the TE-1 cells was not significantly inhibited after 24 hours of continuous irradiation at 8 Gy, compared with cells without continuous irradiation (Figure 2b). These findings imply that miR-21 was associated with radiation resistance in ESCC cells. After cumulative radiation exposure, the cells developed a distinct radioresistant phenotype, with increased expression levels of the tumor radioresistance factors heat shock protein90 and HER3 (data not shown), indicating the establishment of a radioresistant cell line.
Figure 2.
Effects of different radiation doses on miR-21 levels and viability in TE-1 cells. Cells were exposed to different doses (0, 2, 4, 6, 8 Gy) of radiation at a rate of 2.4 Gy/minute, cultured for 48 hours, and then collected. (a) MiR-21 levels were detected by qRT-PCR. (b) After 48 hours irradiation at 8 Gy, irradiation was removed or not. Viability of TE-1 cells was detected by CCK8 assay. *P<0.05, **P<0.01, ***P<0.001 vs. 0 Gy.
Downregulation of miR-21 suppressed proliferation of irradiated ESCC cells via PTEN pathway
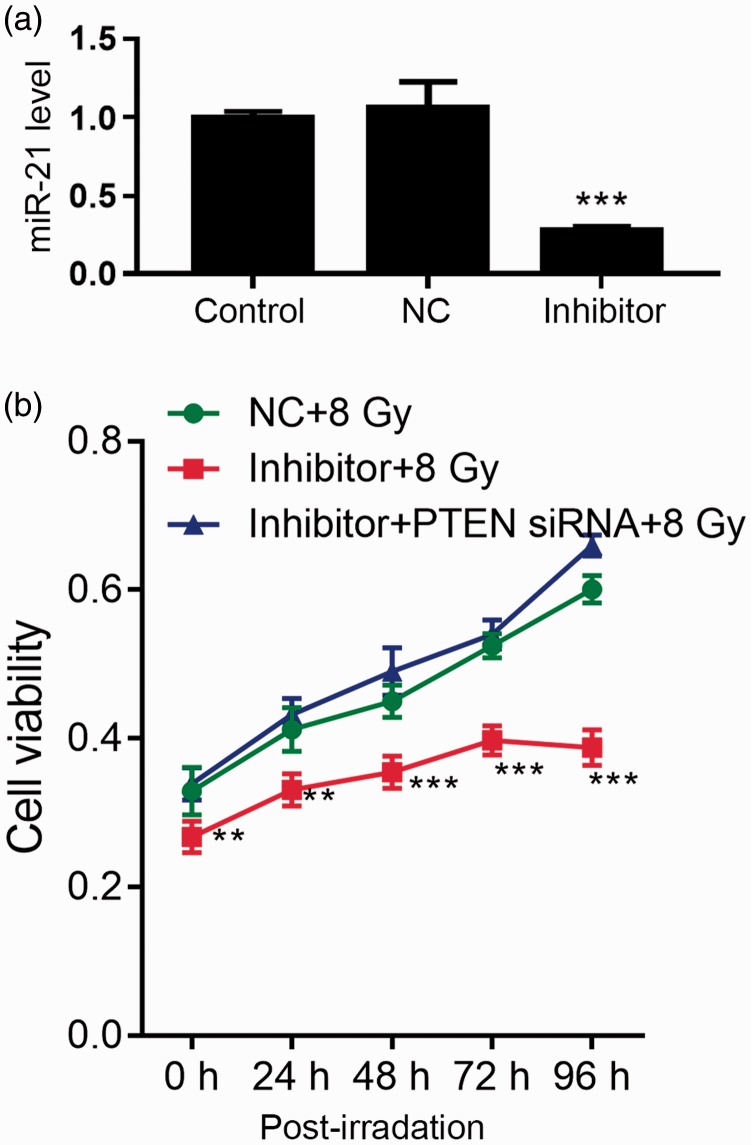
To determine the role of miR-21 in ESCC during irradiation, we transfected ESCC cells with miR-21 inhibitor or NC vector for 48 hours, and confirmed the downregulation of miR-21 by qRT-PCR (Figure 3a).
Figure 3.
Viability of miR-21 inhibitor- or PTEN siRNA-transfected TE-1 cells after exposure to 8 Gy for 48 hours. After transfection, cells were irradiated with 8 Gy for 48 hours, followed by removal of irradiation or not. Cell viability was then detected by CCK8 assay. (a) Expression of miR-21 was detected by qRT-PCR after transfection for 48 hours. ***P<0.001 vs. control. (b) Cell viability. **P<0.01, ***P<0.001 vs. control+8 Gy. NC, negative control.
After transfection for 48 hours, cells were exposed to 8 Gy irradiation at a rate of 2.4 Gy/minute for 48 hours, followed by assessment of cell proliferation (Figure 3b). Downregulation of miR-21 significantly inhibited cell viability under irradiation compared with NC transfection (P<0.05). The effect of miR-21 inhibitor transfection on cell viability was reversed by PTEN siRNA (Figure 3b). These results indicated that miR-21 mediated radiation resistance in ESCC cells via the PTEN pathway.
MiR-21 inhibitor increased expression of cell apoptosis markers via PTEN pathway
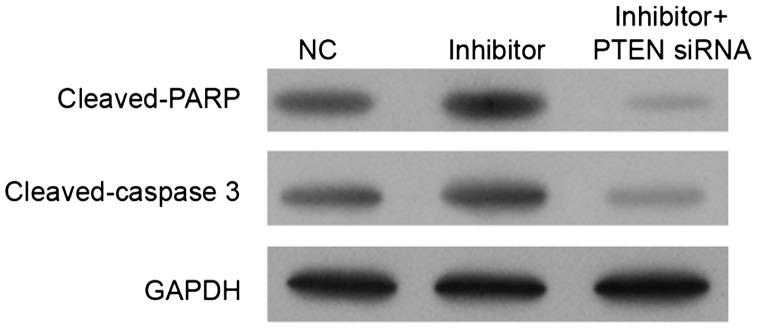
The effects of miR-21 inhibitor and PTEN on cell apoptosis were confirmed by detecting the expression levels of caspase 3 and PARP (Figure 4). After 8 Gy irradiation for 48 hours, miR-21 inhibitor significantly induced PARP and caspase 3 activities (P<0.05), and this effect was reversed by PTEN siRNA. miR-21 inhibition thus increased PARP activity and caspase 3 activity via the PTEN pathway.
Figure 4.
Expression levels of cleaved-caspase 3 and cleaved-PARP in miR-21 inhibitor- and/or PTEN siRNA-transfected TE-1 cells after exposure to 8 Gy for 48 hours. After transfection, cells were irradiated with 8 Gy for 48 hours and then collected, and PTEN, cleaved-caspase 3, and cleaved-PARP levels were detected by western blot. NC, negative control.
Discussion
In the current study, we used an miR-21 inhibition vector and PTEN siRNA to examine the roles of miR-21 and PTEN in the radioresistance of ESCC. We collected pathological specimens from patients with ESCC and divided the patients into radiation-sensitive and radiation-resistant groups based on their response to radiation. We then tested the hypothesis that miRNA-21 promoted radiation resistance of tumor cells by inhibiting the expression of PTEN, both in vivo and in vitro.
Radiation therapy is widely used as a routine clinical treatment for cancer, especially for some tumors with a specific physiological anatomy.23,24 However, interindividual differences result in a relatively high frequency of radiation-induced radioresistance,25 though the mechanism responsible is not yet clear. Recent miRNA research has revealed that radiation affects the expression profiles of miRNAs, and evidence suggests that radiation resistance may be caused by the abnormal expression of single miRNAs.26,27 miRNA-21 was widely overexpressed in solid tumors, associated with a poor tumor prognosis.28 In the present study, we detected miR-21 expression levels in radiosensitive and radioresistant patients with ESCC, and showed that levels were significantly higher in the radiation-resistant group. This result was consistent with previous studies and further corroborates the role of miR-21 in ESCC.21 Overexpression of miR-21 was correlated with the generation of radioresistance, and the current in vitro cell experiments showed that miRNA-21 expression levels increased with increasing radiation dose. Overall, the above experiments suggest that miRNA-21 is related to the generation of radiation resistance in ESCC, and that high expression levels of miRNA-21 can promote radioresistance in ESCC.
Li et al.21reported that inhibition of miR-21 increased the radiosensitivity of esophageal cancer cells by downregulating the expression of PTEN.21PTEN is a tumor suppressor gene located on chromosome 10q23, which is mutated or deleted in a variety of tumors.29,30 The most important substrate for PTEN is phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which is the product of phosphatidylinositol-3 kinase (PI3K) and mediates the activation of Akt (also known as protein kinase B). PTEN dephosphorylates PIP3 to maintain a low level of PIP3, thereby downregulating the PI3K/Akt pathway as part of the PTEN-PI3K/Akt signal transduction pathway.31 Furthermore, PTEN targeting by miR-21 has been assessed by luciferase assay.15,32,33 In this study, we transfected ESCC cells with miR-21 inhibitor or NC vector and found that downregulation of miR-21 significantly inhibited cell viability, but this effect was reversed by PTEN siRNA. We therefore speculate that miRNA-21 induces radioresistance in ESCC by inhibiting PTEN. We further confirmed our hypothesis by examining the expression of apoptotic proteins in the PTEN pathway after irradiation of TE-1 cells with miR-21 inhibitors, and found that miR-21 inhibitors significantly induced PARP activity and caspase 3 activity, and that this inhibition was also reversed by the addition of PTEN siRNA. Overall, the above experimental studies suggest that miR-21 acts via the PTEN pathway in ESCC.
Conclusion
This study demonstrated that miR-21 is abnormally overexpressed in patients with radioresistant ESCC. Furthermore, experimental studies revealed that this radioresistance was achieved by miR-21 regulation of its target gene PTEN. These results thus confirm that miR-21 and its target gene PTEN may be potential molecular targets for the future treatment of patients with ESCC.
Availability of data and materials
The raw data used and analyzed for the current study (not including any personal statements or information) are available from the corresponding author on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Siegel RL, Miller KD, Dvm AJ. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 11. [Google Scholar]
- 2.Chung CS,Lee YC, Wu MS. Prevention strategies for esophageal cancer: perspectives of the East vs West. Best Pract Res Clin Gastroenterol 2015; 29: 15. [DOI] [PubMed] [Google Scholar]
- 3.Berger B, Belka C. Evidence-based radiation oncology: oesophagus. Radiother Oncol 2009; 92: 276–290 [DOI] [PubMed] [Google Scholar]
- 4.Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 5.Houtgraaf JH,Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med 2006; 7: 172. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ., Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 2005; 5: 516–525. [DOI] [PubMed] [Google Scholar]
- 7.Pirollo KF, Hao Z,Rait A, et al. Evidence supporting a signal transduction pathway leading to the radiation-resistant phenotype in human tumor cells. Biochem Biophys Res Commun 1997; 230: 196–201. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry MA. Real-time PCR analysis of micro-RNA expression in ionizing radiation-treated cells. Cancer Biother Radiopharm 2009; 24: 49–56 [DOI] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature 2004; 431: 350–355. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y. Advances in mechanism and treatment strategy of cancer. Cell Mol Biol (Noisy-le-grand) 2018; 64: 1–3. [PubMed] [Google Scholar]
- 11.Yao Y, Rabodzey A, Dewey CF., Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 2007; 293: H1023–H1030. [DOI] [PubMed] [Google Scholar]
- 12.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle 2005; 4: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 14.Wang B., Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol 2012; 138: 1659–1666. [DOI] [PubMed] [Google Scholar]
- 15.Meng F, Henson R,Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007; 133: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JG, Wang JJ,Zhao F, et al. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 2010; 411: 846–852. [DOI] [PubMed] [Google Scholar]
- 17.Bao B, Ali S,Kong D, et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One 2011; 6: e17850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Liu ZL, Wang H,Liu J, et al. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem 2013; 372: 35–45. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Zhu H,Yang X, et al. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumor Biol 2014; 35: 3975–3979. [DOI] [PubMed] [Google Scholar]
- 20.Song L, Liu S,Zhang L, et al. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop and the Akt-mTOR signaling pathway. Tumor Biol 2016; 37: 12161–12168. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Mao WM,Zheng ZG, et al. Down-regulation of PTEN expression modulated by dysregulated miR-21 contributes to the progression of esophageal cancer. Dig Dis Sci 2013; 58: 3483–3493. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Lv JH,Liang L, et al. Downregulation of microRNA-21 inhibited radiation-resistance of esophageal squamous cell carcinoma. Cancer Cell Int 2018; 18: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N, Xia P,Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002; 53: 12–22. [DOI] [PubMed] [Google Scholar]
- 24.Gignoux M, Roussel A,Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study by the EORTC. World J Surg 1987; 11: 426–32. [DOI] [PubMed] [Google Scholar]
- 25.Peters LJ, et al. Keynote address—the problem: Tumor radioresistance in clinical radiotherapy. Int J Radiat Oncol Biol Phys 1981. 8(1): p. 101–108. [DOI] [PubMed] [Google Scholar]
- 26.Chun-Zhi Z,Lei H,An-Ling Z, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010; 10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu C, Liang Z,Huang J, et al. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle 2012; 11: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan LX, Huang XF,Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008; 14: 2348–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J 2004; 382: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steck PA, Pershouse MA,Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362. [DOI] [PubMed] [Google Scholar]
- 31.Paez J., Sellers WR. PI3K/PTEN/Akt Pathway. Cancer Treat Res 2004; 115: 145–167. [PubMed] [Google Scholar]
- 32.Yang Z,Fang S,Di Y, et al. Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. Plos One 2015; 10: e0121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu HY, Li C,Bai WD, et al. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. Plos One 2014; 9: e97114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used and analyzed for the current study (not including any personal statements or information) are available from the corresponding author on reasonable request.