Figure 1:
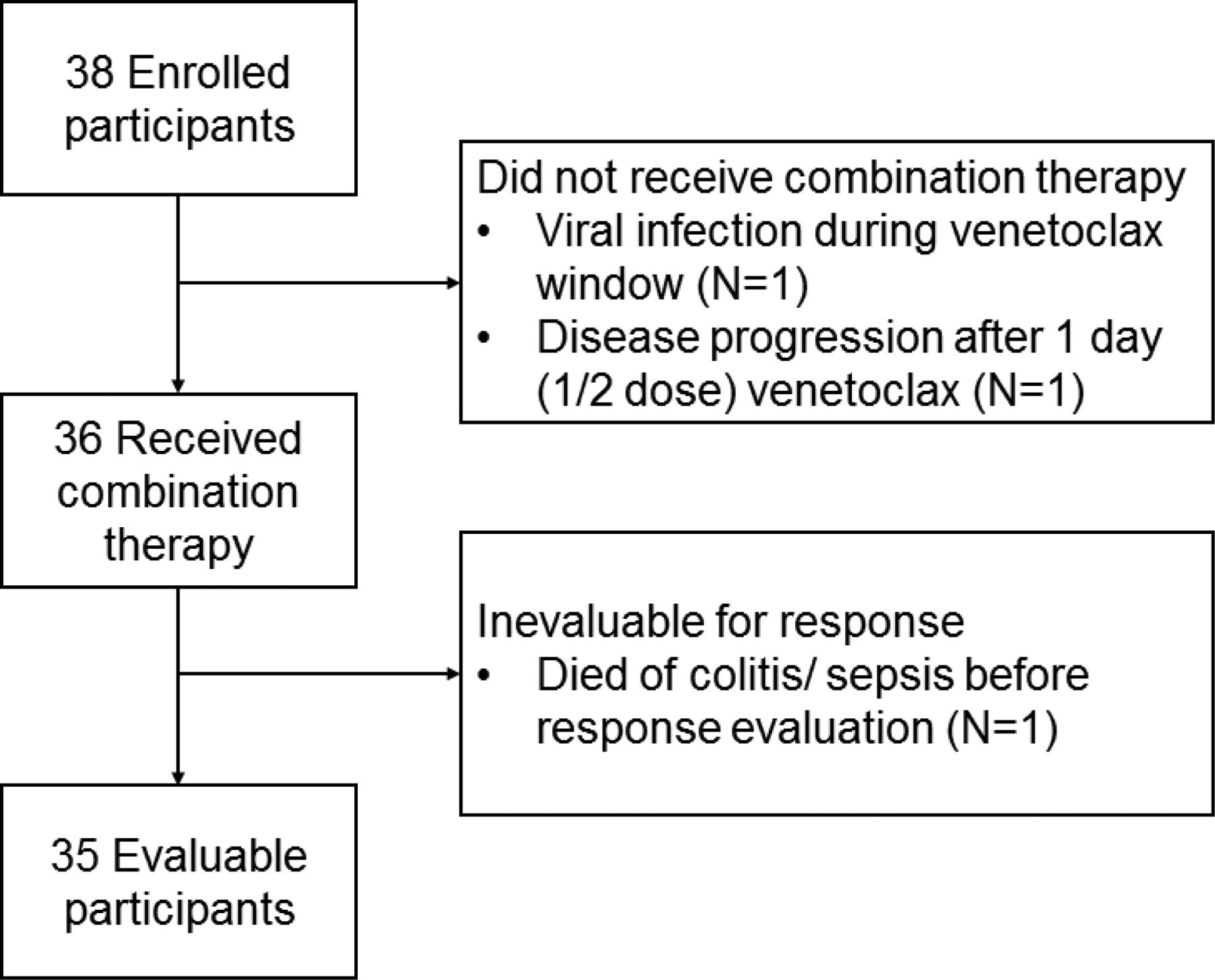
CONSORT diagram of enrolled and analyzed patients Of 38 enrolled participants, 3 were inevaluable for response. Two did not receive combination therapy either due to a viral infection during the venetoclax window (removed so as to avoid myelosuppressive therapy with an active infection) and a patient whose disease progression after the day 1 (1/2 dose venetoclax) precluded ongoing therapy (including the early introduction of chemotherapy as allowed in the protocol; N=1 each). Of 36 patients who received venetoclax and chemotherapy, 1 was inevaluable due to dying of toxicity prior to response evaluation and 35 were evaluable for response.
