Abstract
Circadian rhythms in behavior and physiology are produced by central brain clock neurons that can be divided into subpopulations based on molecular and functional characteristics. It has become clear that coherent behavioral rhythms result from the coordinated action of these clock neuron populations, but many questions remain regarding the organizational logic of the clock network. Here we used targeted genetic tools in Drosophila to eliminate either molecular clock function or neuronal activity in discrete clock neuron subsets. We find that neuronal firing is necessary across multiple clock cell populations to produce free-running rhythms of rest and activity. In contrast, such rhythms are much more subtly affected by molecular clock suppression in the same cells. These findings demonstrate that network connectivity can compensate for a lack of molecular oscillations within subsets of clock cells. We further show that small ventrolateral (sLNv) clock neurons, which have been characterized as master pacemakers under free-running conditions, cannot drive rhythms independent of communication between other cells of the clock network. In particular, we pinpoint an essential contribution of the dorsolateral (LNd) clock neurons, and show that manipulations that affect LNd function reduce circadian rhythm strength without affecting molecular cycling in sLNv cells. These results suggest a hierarchical organization in which circadian information is first consolidated among one or more clock cell populations before accessing output pathways that control locomotor activity.
Keywords: Drosophila, circadian, circuit, sLNv, LNd
Introduction
The circadian timing system produces daily rhythms in behavioral and physiological processes, coordinating these processes with respect to one another and to the external environment. At the core of the circadian system lies a network of interconnected populations of central brain clock neurons that keep time through molecular clocks (Mohawk et al., 2012; Dubowy and Sehgal, 2017). Our understanding of the genetic and molecular underpinnings of the circadian clock has been greatly enhanced by foundational studies undertaken in the fruit fly, Drosophila melanogaster, and fundamental circadian machinery is conserved between flies and mammals (Stanewsky, 2003; Crane and Young, 2014). This extends to similarities between the neuronal circuits through which the central clock controls circadian rhythms, including the importance of neuropeptide signaling for the synchronization of molecularly-distinct clock cell populations that together generate coherent output signals (Dubowy and Sehgal, 2017; Hastings et al., 2018).
In Drosophila, the molecular circadian clock consists of a transcriptional-translational feedback loop based on rhythmic expression of the core clock genes period (per) and timeless (tim). Transcription of per and tim is driven by the transcriptional activators CLOCK (CLK) and CYCLE (CYC). PER and TIM proteins accumulate in the cytoplasm during the day and eventually translocate to the nucleus where they repress their own transcription. Subsequent degradation of these proteins then allows a new round of transcription, with the entire process taking ~24 hours. This molecular clock is present in ~150 central clock neurons in the fly brain, which are organized into subsets based on anatomical and functional properties. These include the large- and small- ventrolateral neurons (lLNvs and sLNvs, respectively), the dorsolateral neurons (LNds), the lateral posterior neurons (LPNs), and three groups of dorsal neurons (DN1s, DN2s, and DN3s) (Figure 1).
Figure 1.
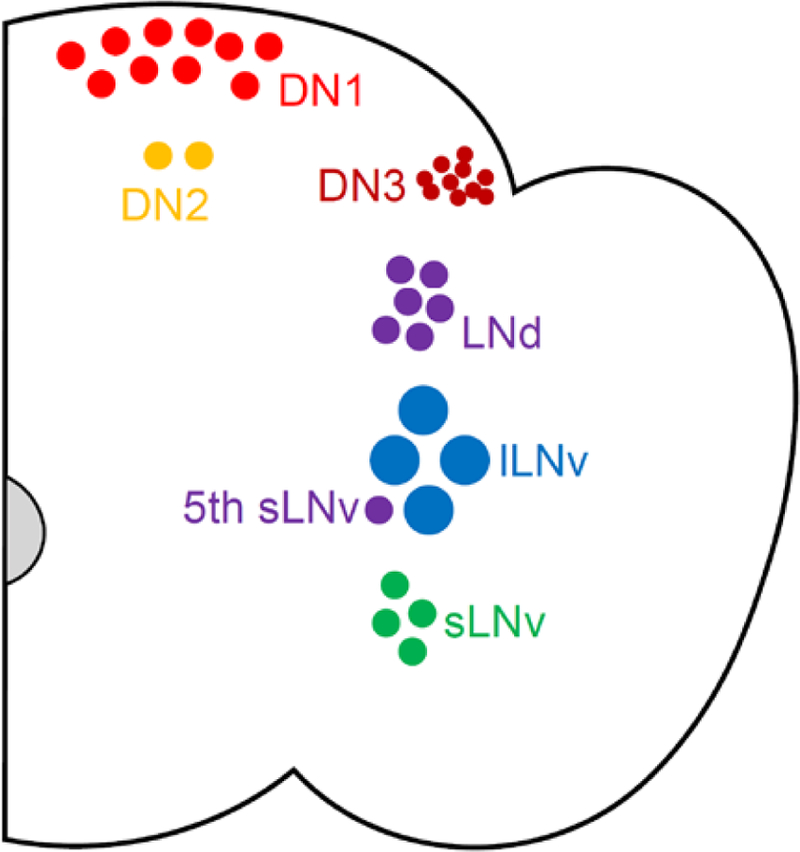
Organization of Drosophila clock cells. A coronal cross-section schematic of a single hemisphere of a Drosophila brain is shown demonstrating small and large ventral lateral neurons (sLNv and lLNv; green and blue, respectively), the 5th sLNv (purple), dorsal lateral neurons (LNd; purple), and dorsal neurons (DN1, DN2, and DN3; light red, orange, and dark red, respectively). This coloring scheme is maintained throughout subsequent figures. Lateral posterior neurons (LPNs) are not shown.
To generate behavioral rhythms, circadian information provided by clock neurons must be coordinated across neuronal populations within and outside the clock network and translated into coherent behavioral outputs. While core clock neurons have been identified in many organisms, questions remain about the organization and hierarchy among these clock cells. Originally, the sLNv neurons were posited as master circadian pacemakers in flies, given that ablation or neuronal silencing of these cells eliminates rest:activity rhythms under conditions of constant darkness (DD) (Renn et al., 1999; Nitabach et al., 2002). Furthermore, PER expression limited to sLNvs appears sufficient to drive behavioral rhythms (Grima et al., 2004). However, more recent work has questioned the central role of the sLNvs, instead favoring a model in which different clock cell populations assume dominance depending on environmental conditions (Picot et al., 2007; Stoleru et al., 2007; Beckwith and Ceriani, 2015; Yao et al., 2016). Whether these populations access independent parallel downstream pathways or whether output information is first consolidated in one or more clock cell populations is yet to be determined.
Here, we sought to inform an understanding of the non-LNv clock cells that are critical for circadian rhythmicity through use of the GAL4-UAS system (Brand and Perrimon, 1993) to prevent either molecular clock cycling or electrical firing within discrete clock cell populations. We show that flies lacking functional clocks in non-LNv cells retain behavioral rhythms but at reduced strength. In contrast, electrically silencing non-LNv clock neurons virtually eliminates rest:activity rhythms. We found similar results when we selectively modulated LNd neurons: electrically silencing these cells produces a substantially more profound reduction in DD rhythm strength than eliminating clock function. Notably, LNd cell manipulations affect behavioral rhythms without altering molecular oscillations in sLNv neurons. These findings show that network interactions can compensate for a lack of clock function in small groups of clock neurons. They also highlight a pivotal role of clock network connectivity in maintaining coherent behavioral rhythms and demonstrate that pacemaker sLNv neurons are unable to drive rhythms in the absence of neuronal communication between other neurons of the clock network. This suggests that information derived from LNv neurons must pass through other clock neurons before accessing output cell populations.
Materials and Methods
Fly Stocks
Flies were raised on cornmeal-molasses medium in light and temperature-controlled incubators held at 25°C and running on a 12:12 light-dark (LD) schedule. The following fly lines were ordered from the Bloomington Drosophila Stock Center: UAS-cycDN (36317), UAS-GFPn (4775), and R78G02-GAL4 (40010). Pdf-GAL4, UAS-Kir2.1 (III) (Baines et al., 2001) and tub-GAL80ts (McGuire et al., 2004) were provided by A. Sehgal. Clk856-GAL4 (Gummadova et al., 2009) was provided by O. Shafer. DvPdf-GAL4 (Bahn et al., 2009), Pdf-GAL80 (II) and Pdf-GAL80 (III) were provided by M. Rosbash. MB122B-GAL4 is a split GAL4 line that was provided by H. Dionne, A. Nern and G. Rubin. More detailed characterization of this driver will be presented elsewhere (manuscript in preparation). Clk4.1-GAL4 (Zhang et al., 2010) was provided by P. Hardin. InSITE911-GAL4 (Gohl et al., 2011) was provided by T. Clandinin. Clk9M-GAL4;Pdf-GAL80 (Kaneko et al., 2012) was provided by F. Hamada. GAL4 and UAS lines were crossed to iso31 flies (Ryder et al., 2004) to generate genetic controls for behavioral and immunohistochemical experiments.
Immunohistochemistry
Adult male fly brains were dissected in cold PBS with 0.1% Triton-X (PBST) and fixed in 4% formaldehyde for 30–60 min at room temperature. Brains were rinsed 3 × 10 min with PBST, blocked for 30–60 min in 5% normal donkey serum (Jackson 017–000-121) in PBST (NDST), and incubated overnight at 4°C in primary antibody diluted in NDST. Brains were then rinsed 3 × 10 min in PBST, incubated 2 hrs in secondary antibody diluted in NDST, rinsed 3 × 10 min in PBST, cleared for 5 min in 50% glycerol in PBST and mounted with Vectashield (Vector Laboratories). Primary antibodies were as follows: rabbit anti-GFP 1:1000 (Molecular Probes A-11122), guinea pig anti-PER 1:1000 (UPR 1140; gift of A. Sehgal), mouse anti-PDF 1:1000 (Developmental Studies Hybridoma Bank PDFC7; generated by J. Blau). Secondary antibodies were as follows: FITC donkey anti-rabbit 1:1000 (Jackson 711–095-152), Cy3 donkey anti-guinea pig 1:1000 (Jackson 706–165-148), Cy5 donkey anti-mouse 1:1000 (Jackson 715–175-151). Immunolabeled brains were visualized with an Olympus Fluoview FV1000 confocal microscope.
GAL4 Expression Characterization
GAL4 lines were crossed to UAS-GFPn (which encodes for a nuclear-localized Green Fluorescent Protein) flies to visualize cells in which GAL4 is functional. Adult male flies were entrained to a 12:12 LD schedule at 25°C for ≥ 5 d. Brains were dissected at ZT23 to ensure prominent PER expression (ZT stands for zeitgeber time, with the time of lights-on corresponding to ZT0), and processed for GFP, PER and Pigment Dispersing Factor (PDF) staining as described above. As analysis of these images was qualitative rather than quantitative, gain and offset settings were optimized for each brain during image capture, and images were adjusted for brightness (Adobe Photoshop) by applying a uniform adjustment across all pixels in the image.
PER Expression Analysis
Adult male flies were entrained to a 12:12 LD cycle at 25°C for ≥ 5 d, then transferred to DD, and staining for PER and PDF was conducted as described above every six hours on either the first or fifth day of DD. Gain and offset were set to minimize saturation, and confocal capture settings were held constant for all brains in a given experiment. We separately quantified PER expression in sLNv, LNd and 5th sLNv neurons. 7–10 brains of each genotype were examined for each time point, and cells were quantified in only one hemisphere from each brain. ImageJ was used to outline the PER+ portion of each clock cell (without regard to whether the signal was localized to the cytoplasm or nucleus) in a single 1 µm confocal section at the center of the cell’s nucleus. Normalized PER intensity was calculated by dividing the mean PER pixel intensity within this area of interest by the mean pixel intensity of the entire confocal frame. These individual clock cell PER intensities were then averaged for all cells of a given population in a brain to determine a single PER staining intensity for each clock cell population in each brain. These data are presented as mean ± standard error measure (SEM) per brain.
sLNvs were identified based on PDF expression and differentiated from lLNvs based on relative location and soma size. We only quantified PER staining in sLNv neurons that we could unequivocally identify as PDF+. Thus, though there should be four PDF+ sLNvs per hemisphere, occasionally fewer than four cells contributed to our analysis for a given brain. LNd and 5th sLNv cells were identified based on anatomical location and lack of PDF expression. Because we did not have an independent marker of these populations, our analysis was based on the assumption that there should be six LNds and one 5th sLNv in each brain hemisphere. In cases where we failed to identify the expected number of PER+ cells for these populations, we assumed that PER levels in any unidentified cells were at background levels, and therefore set the normalized PER intensity for these cells to one.
Images from PER staining are presented without any post-capture image manipulation with the exception of the LNd neurons in Figure 10B. Because PER staining was dim in these brains, we applied a uniform brightness adjustment (Adobe Photoshop) across all pixels of all images shown.
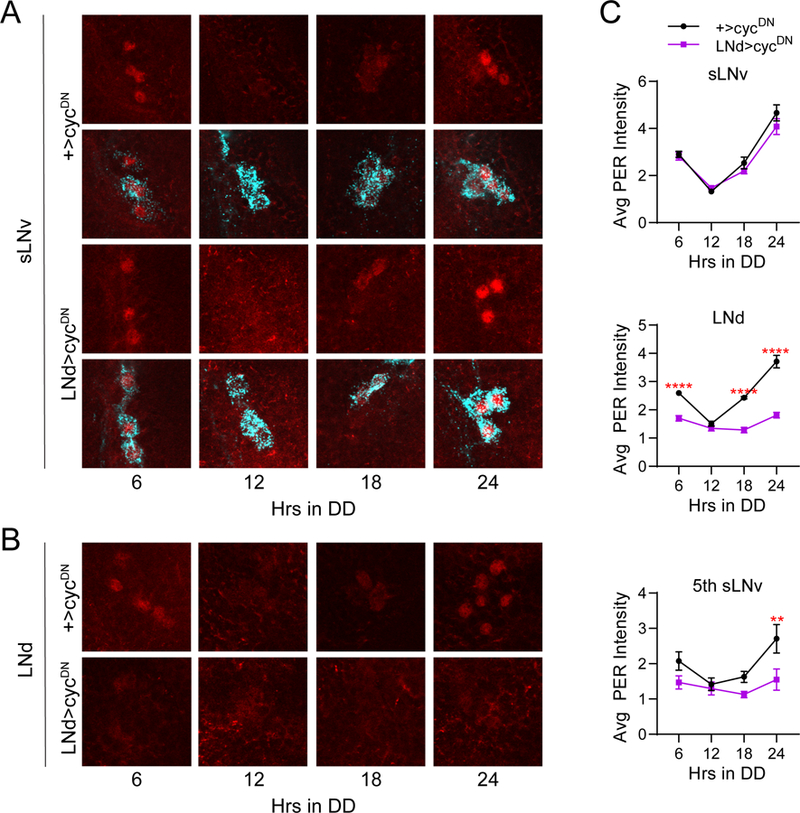
Figure 10.
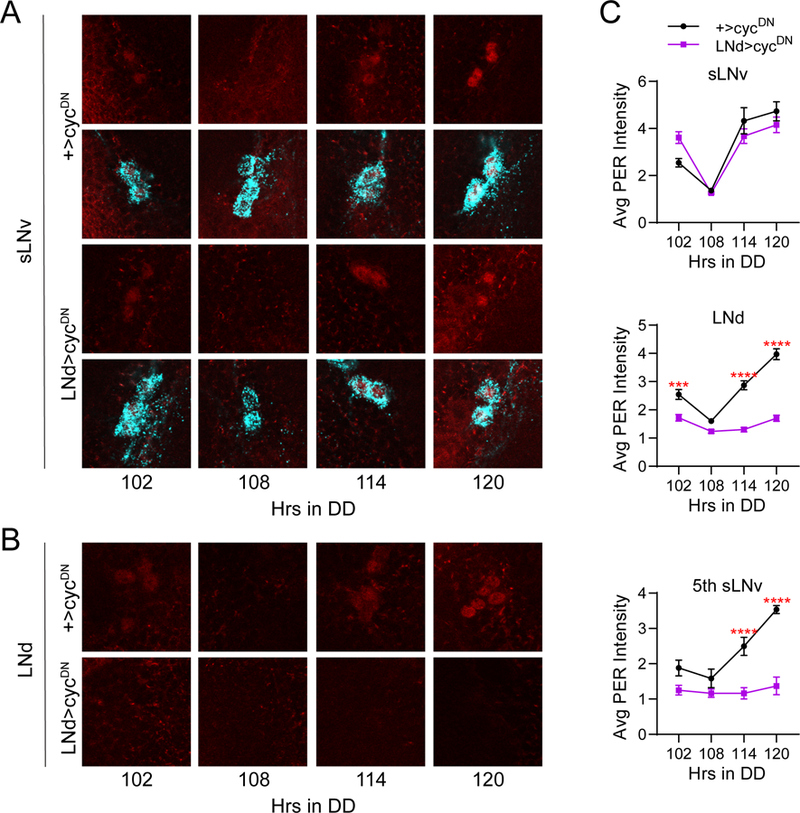
Molecular clock suppression in all six LNd neurons has no effect on LNv clocks. A, Representative confocal images of PER staining (red) in sLNv clock cells at different time points taken every six hours on the fifth day of constant darkness (DD5). PDF staining (cyan) was used to identify sLNv cells, and a merged image of PER and PDF staining is shown below each PER staining panel. PER cycling in sLNvs of LNd>cycDN brains (bottom) was indistinguishable from control +>cycDN brains (top) even after extended time under free-running conditions. B, Representative confocal images of PER staining (red) in LNd cells taken from the same genotypes and time points as in A. PER levels and cycling are strongly suppressed in LNd neurons of LNd>cycDN flies (bottom) compared to control brains (top), which exhibited normal PER cycling. C, Quantification of average PER staining intensity, normalized to background levels (mean ± SEM), for sLNv cells (top), LNd cells (middle) and the 5th sLNv (bottom). n= 8 brains per time point; ****p<0.0001 compared to control PER intensity at that time point, Sidak’s multiple comparisons test following Two-Way ANOVA.
Rest:Activity Rhythm Analysis
Prior to behavioral experiments, flies were entrained to a 12:12 LD cycle at 25°C for ≥ 5 d. Following entrainment, individual ~7 d old male flies were loaded into glass tubes containing 5% sucrose and 2% agar for locomotor activity analysis with the Drosophila Activity Monitoring System (Trikinetics, Waltham MA). DAMS monitoring was conducted at 25°C in DD conditions and data were acquired every minute. Rest:activity rhythm period and strength (power) were analyzed for days 2–7 of DD with ClockLab software (Actimetrics, Wilmette IL) using chi-squared periodogram analysis. Rhythm power is defined as the amplitude of the periodogram line at the dominant period minus the chi-squared significance line (at a significance of p<0.01).
Flies that died during the course of behavioral monitoring were identified via visual inspection of activity records and removed from analysis. All flies that survived through the end of the one-week monitoring period were included in mean rest:activity power determination. Because rhythm strength cannot be negative, flies with a calculated power <0 were assigned a power of 0 for subsequent analysis. Only flies with a power >0 were included in determination of mean rest:activity rhythm period. We conducted at least two independent behavioral experiments for each genotype and pooled data from separate experiments for analysis. Representative individual activity records displayed in Figures 3B, 7B, 8B, 8D, 9B and 9D were selected to reflect the mean rhythm strength of a given genotype. Thus, we chose individual records that displayed a rhythm power that fell within the standard error of the means listed in Table 1.
Figure 3.
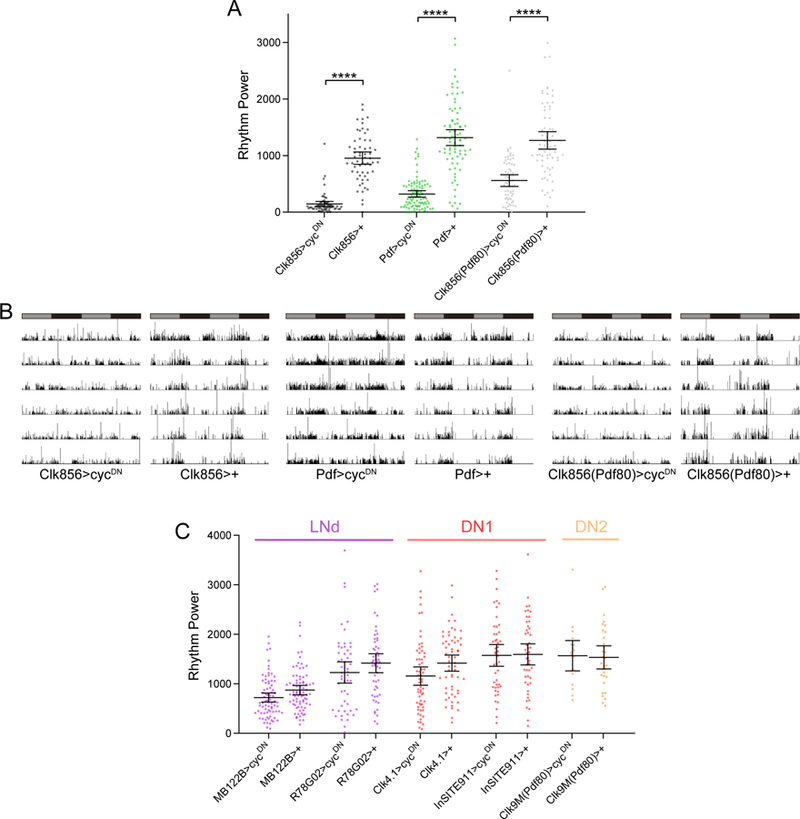
Molecular clocks in non-LNv clock cells are required for robust free-running rest:activity rhythms. A, Rest:activity rhythm power is displayed for the genotypes listed. See methods for a description of determination of rhythm power. Lines are means ± 95% confidence intervals. Dots represent individual flies. n=59–91 per genotype; ****p<0.0001, Tukey HSD test following ANOVA. See Table 1 for exact n and p values. B, Representative single fly activity records over six days in DD for the genotypes listed. Activity in infrared beam breaks/min is plotted for each minute. Activity records are double plotted, with 48 hours of data on each line and the second 24 hours replotted at the start of the next line. Gray and black bars above each plot represent subjective day and night, respectively. C, Rest:activity rhythm power is displayed for the genotypes listed and plotted as in A. n=18–79 per genotype. Note that rest:activity rhythm strength is unaffected by selective molecular clock abrogation in specific non-LNv subsets of clock neurons.
Figure 7.
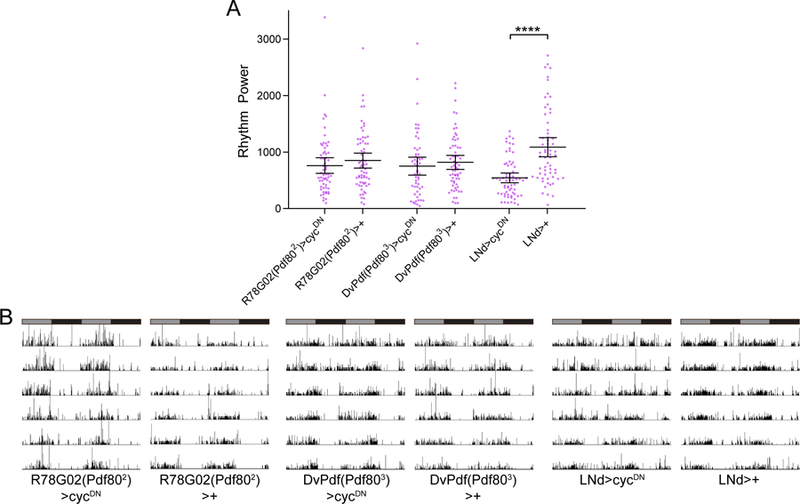
Molecular oscillations in all six LNd neurons are required for robust free-running rest:activity rhythms. A, Rest:activity rhythm power is displayed for the genotypes listed. Lines are means ± 95% confidence intervals. Dots represent individual flies. n=53–64 per genotype; ****p<0.0001, Tukey HSD test following ANOVA. See Table 1 for exact n and p values. Rhythm strength was unaffected by molecular clock abrogation in either the CRY+ (R78G02(Pdf802)>cycDN) or CRY- (DvPdf(Pdf803>cycDN) LNds individually; however, collective clock suppression in all six LNd neurons (LNd>cycDN) moderately but significantly reduced rhythm strength. B, Representative single fly activity records over six days in DD for the genotypes listed. Activity in infrared beam breaks/min is plotted for each minute. Activity records are double plotted and gray and black bars above each plot represent subjective day and night, respectively.
Figure 8.
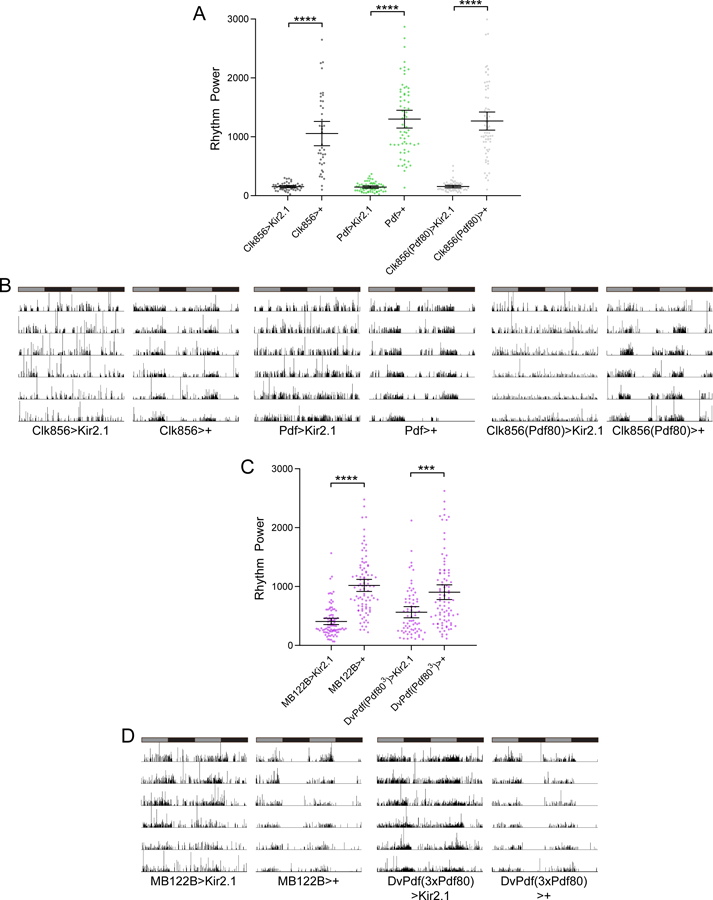
Silencing neuronal activity in non-LNv clock neurons drastically degrades free-running rest:activity rhythms. A, Rest:activity rhythm power is displayed for the genotypes listed. Lines are means ± 95% confidence intervals. Dots represent individual flies. n=40–67 per genotype; ****p<0.0001, Tukey HSD test following ANOVA. See Table 1 for exact n and p values. Rhythm strength was strongly and equivalently reduced following neuronal silencing of all clock neurons (Clk856>Kir2.1), sLNv neurons (Pdf>Kir2.1), or non-LNv clock neurons (Clk856(Pdf80)>Kir2.1). B, Representative single fly activity records over six days in DD for the genotypes listed. Activity in infrared beam breaks/min is plotted for each minute. Activity records are double plotted and gray and black bars above each plot represent subjective day and night, respectively. C, Rest:activity rhythm power is displayed for the genotypes listed as described for A. n=71–91 per genotype; ****p<0.0001, *** p<0.001 Tukey HSD test following ANOVA. See Table 1 for exact n and p values. Silencing of either the CRY+ (MB122B>Kir2.1) or the CRY- (DvPdf(Pdf803)>Kir2.1) subset of LNd neurons reduced rest:activity rhythm strength. D, Representative single fly activity records for the genotypes listed are plotted as in B.
Figure 9.
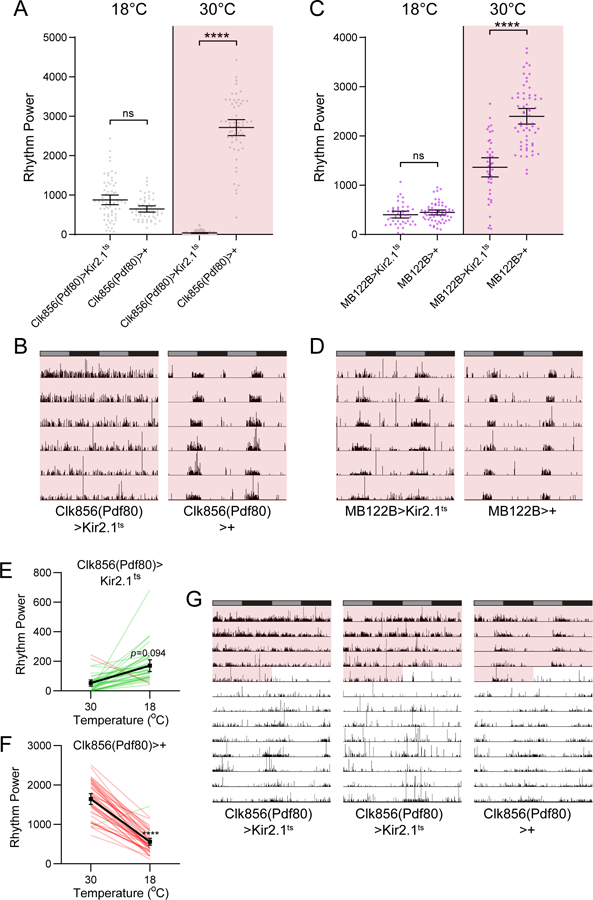
Adult-specific neuronal silencing of non-LNv clock neurons drastically degrades free-running rest:activity rhythms. A, Rest:activity rhythm power is displayed for the genotypes and temperatures listed. Red shading indicates that behavioral testing was conducted at 30°C, which allows for GAL4-mediated expression of Kir2.1. Lines are means ± 95% confidence intervals. Dots represent individual flies. n=53–79 per genotype; ****p<0.0001, Tukey HSD test following ANOVA. See Table 1 for exact n and p values. Rhythm strength was strongly reduced following adult-specific neuronal silencing of all non-LNv clock neurons (Clk856(Pdf80)>Kir2.1ts). B, Representative single fly activity records over six days in DD for the genotypes listed at 30°C. Activity in infrared beam breaks/min is plotted for each minute. Activity records are double plotted and gray and black bars above each plot represent subjective day and night, respectively. C, Rest:activity rhythm power is displayed for the genotypes and temperatures listed as described for A. n=41–60 per genotype; ****p<0.0001, Tukey HSD test following ANOVA. See Table 1 for exact n and p values. Adult-specific silencing of the CRY+ (MB122B>Kir2.1ts) subset of LNd neurons reduced rest:activity rhythm strength. D, Representative single fly activity records for the genotypes and temperature listed are plotted as in B. E and F, Rest:activity rhythm power is displayed for Clk856(Pdf80)>Kir2.1ts (E) and Clk856(Pdf80)>+ (F) flies. Red and green lines represent rhythm power of individual flies assessed at 30°C followed by 18°C. Red lines indicate flies in which power decreased from following transition to 18°C, and green lines indicate flies in which power increased. Black squares represent means ± 95% confidence intervals. n = 36–37 per genotype. ****p<0.0001, Sidak’s multiple comparison test following 2-way Repeated Measures ANOVA. G, Representative single fly activity records for the indicated genotypes showing 5 days at 30°C (pink shading) followed by 9 days at 18°C as plotted in B. Note that Clk856(Pdf80)>Kir2.1ts flies recover rhythmic behavior after a few days at 18°C.
Table 1.
DD Period and Rhythm Strength Following Clock Cell Manipulations. Full genotype, abbreviated name, clock cell expression, number of animals, mean period length ± SEM and mean rhythm power ± SEM are listed for each clock cell manipulation. Mean rhythm power for each experimental line was compared to corresponding GAL4 and UAS controls with ANOVA followed by Tukey HSD posthoc test. Italicized lines are significantly different from both genetic controls at p<0.05.
| Genotype | Name | Clock Cell Expression | n | Period ± SEM | Power ± SEM | Tukey HSD p-values |
|---|---|---|---|---|---|---|
| ----cycDN experiments---- | ||||||
| UAS-cycDN/+ | +>cycDN | -- | 299 | 23.34 ± 0.04 | 1847.8 ± 43.2 | |
| Clk856-GAL4/UAS-cycDN | Clk856>cycDN | All clock cells except some DN3s | 66 | 24.88 ± 0.60 | 144.2 ± 22.0**** | to cycDN: <1.0E-15 to GAL4: 2.0E-14 |
| Clk856-GAL4/+ | Clk856>+ | -- | 59 | 23.62 ± 0.03 | 953.6 ± 54.7 | |
| Pdf-GAL4/UAS-cycDN | pdf>cycDN | LNvs | 91 | 23.91 ± 0.30 | 320.4 ± 28.9**** | to cycDN: 4.4E-14 to GAL4: 5.3E-14 |
| Pdf-GAL4/+ | pdf>+ | -- | 80 | 23.93 ± .010 | 1317.5 ± 70.7 | |
| Clk856-GAL4/UAS-cycDN; Pdf- GAL80/+ | Clk856(Pdf80)> cycDN | All clock cells except LNvs and some DN3s | 63 | 23.77 ± .018 | 557.2 ± 51.9**** | to cycDN: <1.0E-15 to GAL4: 3.1E-09 |
| CLK856-GAL4/+; Pdf-GAL80/+ | Clk856(Pdf80)>+ | -- | 67 | 23.64 ± 0.03 | 1267.6 ± 77.3 | |
| MB122B-AD/UAS-cycDN; MB122-DBD/+ | MB122B>cycDN | 3 CRY+ LNds; 5th sLNv | 78 | 23.36 ± 0.04 | 722.9 ± 46.4 | to cycDN: 1.6E-13 to GAL4: 0.203 |
| MB122-AD/+; MB122-DBD/+ | MB122B>+ | -- | 79 | 23.56 ± 0.09 | 874.0 ± 47.3 | |
| UAS-cycDN/+; R78G02-GAL4/+ | R78G02>cycDN | 3 CRY+ LNds; 5th sLNv | 52 | 23.50 ± 0.09 | 1229.9 ± 107.1 | to cycDN: 1.5E-3 to GAL4: 0.387 |
| R78G02-GAL4/+ | R78G02>+ | -- | 54 | 23.72 ± 0.03 | 1417.3 ± 95.3 | |
| UAS-cycDN/+; Clk4.1-GAL4/+ | Clk4.1>cycDN | 8–10 DN1p | 62 | 23.83 ± 0.04 | 1158.0 ± 92.5 | to cycDN: 7.5E-05 to GAL4: 0.114 |
| Clk4.1-GAL4/+ | Clk4.1>+ | -- | 59 | 23.69 ± 0.05 | 1419.0 ± 82.1 | |
| UAS-cycDN/+; InSITE911-GAL4/+ | InSITE911>cycDN | All DN1ps | 47 | 23.66 ± 0.05 | 1574.8 ± 110.0 | to cycDN: 0.111 to GAL4: 0.991 |
| +/+; InSITE911-GAL4/+ | InSITE911>+ | -- | 48 | 23.76 ± 0.04 | 1594.5 ± 105.8 | |
| Clk9M-GAL4/ UAS-cycDN; Pdf- GAL80/+ | Clk9M(Pfd80) >cycDN | DN2s | 18 | 23.31 ± 0.07 | 1568.3 ± 144.8 | to cycDN: 1.31E-2 to GAL4: 0.983 |
| Clk9M-GAL4/+; Pdf-GAL80/+ | Clk9M(Pdf80)>+ | -- | 31 | 23.45 ± 0.06 | 1534.5 ± 113.8 | |
| UAS-cycDN/+; Pdf-GAL80/+ | +(Pdf80)>cycDN | -- | 133 | 23.57 ± 0.02 | 1729.4 ± 59.7 | |
| UAS-cycDN/+; R78G02-GAL4,Pdf-GAL80/Pdf-GAL80 | R78G02(Pdf802)> cycDN | 3 CRY+ LNds; 5th sLNv | 61 | 23.62 ± 0.04 | 760.8 ± 69.5 | to cycDN: <1.0E-15 to GAL4: 0.703 |
| R78G02-GAL4,Pdf-GAL80/Pdf-GAL80 | R78G02(Pdf802)> + | -- | 64 | 23.65 ± 0.11 | 849.5 ± 66.3 | |
| DvPdf-GAL4,Pdf-GAL80/UAS-cycDN; Pdf-GAL80/Pdf-GAL80 | DvPdf(Pdf803)> cycDN | 3 CRY- LNds; 1 CRY+ LNd; 5th sLNv | 53 | 24.32 ± 0.17 | 750.3 ± 79.6 | to cycDN: <1.0E-15 to GAL4: 0.831 |
| DvPdf-GAL4,Pdf-GAL80/+; Pdf-GAL80/Pdf-GAL80 | DvPdf(Pdf803)>+ | -- | 63 | 23.87 ± 0.05 | 817.3 ± 61.7 | |
| DvPdf-GAL4,Pdf-GAL80/UAS-cycDN; R78G02-GAL4,Pdf- GAL80/Pdf-GAL80 | LNd>cycDN | All LNds; 5th sLNv | 64 | 23.45 ± 0.12 | 543.5 ± 43.1**** | to cycDN: 9.7E-14 to GAL4: 4.7E-06 |
| Dvpdf-GAL4,pdf-GAL80/+; R78G02-GAL4,pdf-GAL80/Pdf-GAL80 | LNd>+ | -- | 63 | 23.67 ± 0.03 | 1085.5 ± 84.6 | |
| DvPdf-GAL4/UAS-cycDN; Pdf-GAL80/+ | DvPdf(Pdf80)> cycDN | Four LNds; LNvs | 48 | 23.83 ± 0.32 | 464.5 ± 60.5**** | to cycDN: 2.0E-15 to GAL4: 8.1E-11 |
| Dvpdf-GAL4/+; Pdf-GAL80/+ | DvPdf(Pdf80)>+ | -- | 47 | 24.26 ± 0.05 | 1329.9 ± 100.6 | |
| DvPdf-GAL4,Pdf-GAL80/UAS-cycDN; Pdf-GAL80/+ | DvPdf(Pdf802)> cycDN | Four LNds; LNvs | 38 | 23.49 ± 0.40 | 422.3 ± 73.4**** | to cycDN: 8.1E-14 to GAL4: 8.6E-07 |
| DvPdf-GAL4,Pdf-GAL80/+; Pdf-GAL80/+ | DvPdf(Pdf802)>+ | -- | 43 | 24.01 ± 0.05 | 1197.3 ± 106.3 | |
| ----Kir2.1 experiments---- | ||||||
| UAS-Kir2.1/+ | +>Kir2.1 | -- | 108 | 23.71 ± 0.04 | 1102.7 ± 59.1 | |
| Clk856-GAL4/+; UAS-Kir2.1/+ | Clk856>Kir2.1 | All clock cells except some DN3s | 50 | 25.30 ± 0.61 | 153.5 ± 8.7**** | to Kir2.1: <1.0E-15 to GAL4: 5.0E-15 |
| Clk856-GAL4/+ | Clk856>+ | -- | 40 | 23.68 ± 0.05 | 1055.8 ± 101.9 | |
| Pdf-GAL4/+; UAS-Kir2.1/+ | Pdf>Kir2.1 | LNvs | 62 | 22.84 ± 0.41 | 146.0 ± 9.9**** | to Kir2.1: 1.5E-14 to GAL4: 5.0E-15 |
| Pdf-GAL4/+ | Pdf>+ | -- | 64 | 23.95 ± 0.06 | 1300.1 ± 76.2 | |
| Clk856-GAL4/+; UAS-Kir2.1/Pdf-GAL80 | Clk856(Pdf80)> Kir2.1 | All clock cells except LNvs and some DN3s | 63 | 24.87 ± 0.38 | 155.2 ± 10.3**** | to Kir2.1: <1.0E-15 to GAL4: <1.0E-15 |
| Clk856-GAL4/+; Pdf-GAL80/+ | Clk856(Pdf80)>+ | -- | 67 | 23.64 ± 0.03 | 1267.6 ± 77.3 | |
| MB122B-AD/+; MB122B-DBD/UAS-Kir2.1 | MB122B>Kir2.1 | 3 CRY+ LNds; 5th sLNv | 91 | 24.16 ± 0.13 | 406.5 ± 27.1**** | to Kir2.1: 3.3E-13 to GAL4: 3.5E-13 |
| MB122B-AD/+; MB122B-DBD/+ | MB122B>+ | -- | 88 | 23.61 ± 0.02 | 1018.3 ± 51.8 | |
| UAS-Kir2.1,Pdf-GAL80/+ | +(Pdf80)>Kir2.1 | -- | 92 | 23.95 ± 0.02 | 1090.4 ± 56.4 | |
| DvPdf-GAL4,Pdf-GAL80/+; UAS-Kir2.1,Pdf-GAL80/Pdf-GAL80 | DvPdf(Pdf803)> Kir2.1 | 3 CRY- LNds; 1 CRY+ LNd; 5th sLNv | 71 | 24.49 ± 0.09 | 563.7 ± 47.8*** | to Kir2.1: 3.5E-09 to GAL4: 1.9E-04 |
| DvPdf-GAL4,Pdf-GAL80/+; Pdf-GAL80 | DvPdf(Pdf803)>+ | -- | 90 | 23.82 ± 0.03 | 903.6 ± 62.7 | |
| ----tubGAL80ts; Kir2.1 experiments---- | ||||||
| tub-GAL80ts/+; UAS-Kir2.1/+ 18°C | +>Kir2.1ts | -- | 53 | 23.73 ± 0.14 | 864.7 ± 56.3 | |
| Clk856-GAL4/tub-GAL80ts; UAS-Kir2.1/Pdf-GAL80 18°C | Clk856(Pdf80)> Kir2.1ts | All clock cells except LNvs and some DN3s | 67 | 23.74 ± 0.17 | 877.9 ± 61.9 | to Kir2.1: 0.999 to GAL4: 0.159 |
| Clk856-GAL4/+; Pdf-GAL80/+ 18°C | Clk856(Pdf80)>+ | -- | 53 | 23.61 ± 0.10 | 646.9 ± 39.6 | |
| MB122B-AD/tub-GAL80ts; MB122B-DBD/UAS-Kir2.1 18°C | MB122B>Kir2.1ts | 3 CRY+ LNds; 5th sLNv | 42 | 24.18 ± 0.24 | 402.3 ± 33.9 | to Kir2.1: 4.3E-04 to GAL4: 0.998 |
| MB122B-AD/+; MB122B-DBD/+ 18°C | MB122B>+ | -- | 59 | 23.67 ± 0.09 | 449.6 ± 25.0 | |
| tub-GAL80ts/+; UAS-Kir2.1/+ 30°C | +>Kir2.1ts | -- | 58 | 23.86 ± 0.07 | 1985.2 ± 103.1 | |
| Clk856-GAL4/tub-GAL80ts; UAS-Kir2.1/Pdf-GAL80 30°C | Clk856(Pdf80)> Kir2.1ts | All clock cells except LNvs and some DN3s | 79 | 22.45 ± 0.44 | 37.6 ± 6.0**** | to Kir2.1: <1.0E-15 to GAL4: <1.0E-15 |
| Clk856-GAL4/+; Pdf-GAL80/+ 30°C | Clk856(Pdf80)>+ | -- | 58 | 23.38 ± 0.03 | 2713.1 ± 101.5 | |
| MB122B-AD/tub-GAL80ts; MB122B-DBD/UAS-Kir2.1 30°C | MB122B>Kir2.1ts | 3 CRY+ LNds; 5th sLNv | 41 | 23.79 ± 0.14 | 1363.7 ± 97.0**** | to Kir2.1: 2.9E-07 to GAL4: 1.0E-11 |
| MB122B-AD/+; MB122B-DBD/+ 30°C | MB122B>+ | -- | 60 | 23.62 ± 0.03 | 2398.9 ± 79.2 | |
p<0.0001
p<0.001 compared to both genetic controls. Specific Tukey HSD p-values are listed.
For adult inducible neuronal silencing, we used a temperature sensitive tub-GAL80 construct (McGuire et al., 2004) to allow temporal control over Kir2.1 expression. To test for effects of adult-specific silencing, flies were raised at 18°C under 12:12 LD conditions and then separate groups were loaded into DAMs and behavioral testing was conducted in DD conditions at either 18°C or 30°C. Rest:activity rhythm period and power were determined as described above for days 2–7 of DD. To test for reversibility of the effects of adult-specific silencing, flies were raised at 18°C under 12:12 LD conditions, then loaded into DAMs in DD conditions and subjected to 5 days of 30°C followed by 9 days of 18°C. Rest:activity rhythm period and power were determined as described above for days 2–5 of 30°C and days 4–8 of 18°C.
Experimental Design and Statistical Analysis
Statistical analysis was performed with GraphPad Prism 7.04 software (La Jolla, CA). 2-Way ANOVA was used to compare PER staining intensities between experimental and control genotypes at different DD time points. Sidak’s multiple comparisons posthoc test was used to test for differences in PER intensity between experimental and control PER staining at each individual time point. ANOVA with Tukey HSD posthoc test was used to compare experimental and control fly rest:activity rhythm powers. 2-way repeated measures ANOVA was used to test for reversibility of adult-specific silencing (Figure 9E and F). Sidak’s multiple comparisons posthoc test was used to assess differences between genotypes at each temperature and within genotypes at different temperatures. Each experimental line was compared to two genetic controls: the GAL4 control, which consisted of the GAL4 line (along with any associated GAL80 constructs) crossed to the iso31 stock, and the UAS control, which consisted of the UAS line crossed to the iso31 stock. In cases where experimental lines contained three copies of GAL80, corresponding UAS controls also contained a copy of the GAL80. Specific genotypes are listed in Table 1. Controls were always run alongside experimental lines. Some genotypes (e.g. +>UAS-cycDN flies) were common across multiple experimental lines. For these lines, the period and power listed in Table 1 represents the means of all flies from all experiments; however, for statistical analysis, each experimental line was compared only to the subset of control flies that were run concurrently. For all analysis, p<0.05 was considered significant.
Results
Molecular Clock Cycling in Non-LNv Clock Neurons is Necessary for Robust DD Rhythms
Others have assessed the sufficiency of molecular clock function in different groups of clock neurons through targeted rescue experiments in which the period gene was selectively restored to discrete clock cell populations (Ewer et al., 1992; Frisch et al., 1994; Vosshall and Young, 1995; Veleri et al., 2003; Grima et al., 2004; Stoleru et al., 2004; Picot et al., 2007; Rieger et al., 2009; Zhang et al., 2010b). In general, these studies showed that free-running behavioral rhythms require PER expression in lateral clock cells, and furthermore concluded that sLNv and LNd neurons drive behavioral rhythms under DD and LL conditions, respectively. In contrast to these studies, we chose to address the question of necessity by using the GAL4-UAS system to drive expression of a dominant-negative CYCLE protein (cycDN) (Tanoue et al., 2004) to eliminate molecular clock function in selective subsets of clock neurons. To confirm the effectiveness and specificity of the cycDN construct, we first used the Pdf-GAL4 line, which expresses in LNv clock neurons. In these flies, we conducted immunohistochemical staining for the PER protein at various time points to test the ability of cycDN to selectively dampen free-running molecular oscillations. As expected, we found that PER expression in the sLNvs of Pdf>cycDN flies (to simplify nomenclature, Pdf>cycDN refers to flies in which Pdf-GAL4 has been used to drive expression of UAS-cycDN; see Table 1 for naming conventions) was constitutively low and failed to oscillate rhythmically throughout the day (Figure 2A and C). As cycDN was limited to PDF+ cells and our PER assessment was conducted on the first day of DD, this manipulation had no obvious effect on PER expression or cycling in LNd cells (Figure 2B and C). Likewise, it did not alter PER expression in the PDF- 5th sLNv (Figure 2C), which displays functional properties often associated with the LNd neurons (Rieger et al., 2006). Consistent with previous findings (Tanoue et al., 2004), abrogation of molecular clock cycling in LNv clock cells also strongly decreased rest:activity rhythm strength (Table 1; Figure 3A and B), confirming the importance of the LNv cells in driving coherent DD rhythms (Renn et al., 1999; Nitabach et al., 2002).
Figure 2.
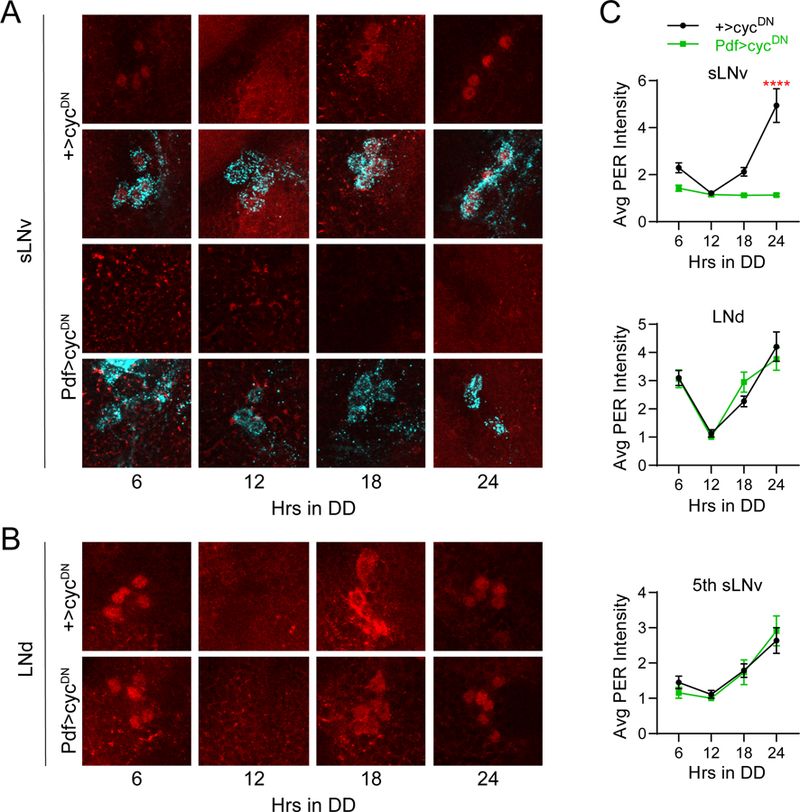
Selective suppression of molecular clock cycling in PDF+ LNvs. A, Representative confocal images of PER staining (red) in sLNv clock cells at different time points taken every six hours on the first day of constant darkness (DD1). PDF staining (cyan) was used to identify sLNv cells, and a merged image of PER and PDF staining is shown below each PER staining panel. Control +>UAS-cycDN brains (top) exhibit normal PER oscillations in LNv neurons, while PER expression in Pdf>cycDN brains (bottom) is constitutively low. B, Representative confocal images of PER staining (red) in LNd cells taken from the same genotypes and time points as in A. PER levels and cycling are unaffected in LNds of Pdf>cycDN flies. C, Quantification of average PER staining intensity, normalized to background levels (mean ± SEM), for sLNv cells (top), LNd cells (middle) and the 5th sLNv (bottom). n= 7–10 brains per time point; ****p<0.0001 compared to control PER intensity at that time point, Sidak’s multiple comparisons test following Two-Way ANOVA.
Having established our genetic system, we next conducted experiments to determine whether molecular oscillations in non-LNv clock neurons are necessary for behavioral rhythms in DD. We began by driving cycDN expression broadly using the Clk856-GAL4 driver (Gummadova et al., 2009), which is expressed in nearly all central clock neurons except some DN3 cells. As these flies lack functional molecular clocks in the vast majority of clock neurons, this resulted in a drastic decrease in rest:activity rhythm strength (Table 1; Figure 3A and B). This manipulation also established a baseline of arrhythmicity against which other manipulations could be compared. For example, though the aforementioned Pdf>cycDN flies have an average power that is strongly reduced relative to control lines, this power is still significantly greater than that of Clk856>cycDN flies (p=0.000992, Tukey HSD test). This suggests that some residual DD rhythmicity can be maintained in the absence of LNv clocks, at least in a subset of flies, which is consistent with previous studies that showed that a small proportion of flies in which LNvs have been genetically ablated or made to lack functional clocks retain low-power rhythms, especially upon initial transfer to DD (Renn et al., 1999; Picot et al., 2007).
The contribution of non-LNv clock neurons to DD rhythms is evidenced by the fact that rest:activity rhythm strength was moderately but significantly reduced when we eliminated clock oscillations in all non-LNv clock neurons by using a combination of the Clk856-GAL4 driver and Pdf-GAL80 (Stoleru et al., 2004) (Table 1; Figure 3A and B). GAL80 suppresses GAL4 function, and we confirmed that a single copy of Pdf-GAL80 was sufficient to completely prevent Clk856-GAL4 from driving expression of a highly sensitive nuclear GFP (GFPn) in LNv clock cells (Figure 4A-C), and also to prevent cycDN-mediated suppression of PER expression in these cells (Figure 4D and E). Although many Clk856(Pdf80)>cycDN flies retained appreciable activity rhythms, they were consistently weaker than control lines and were characterized by less consolidated periods of rest and activity (Figure 3A and B).
Figure 4.
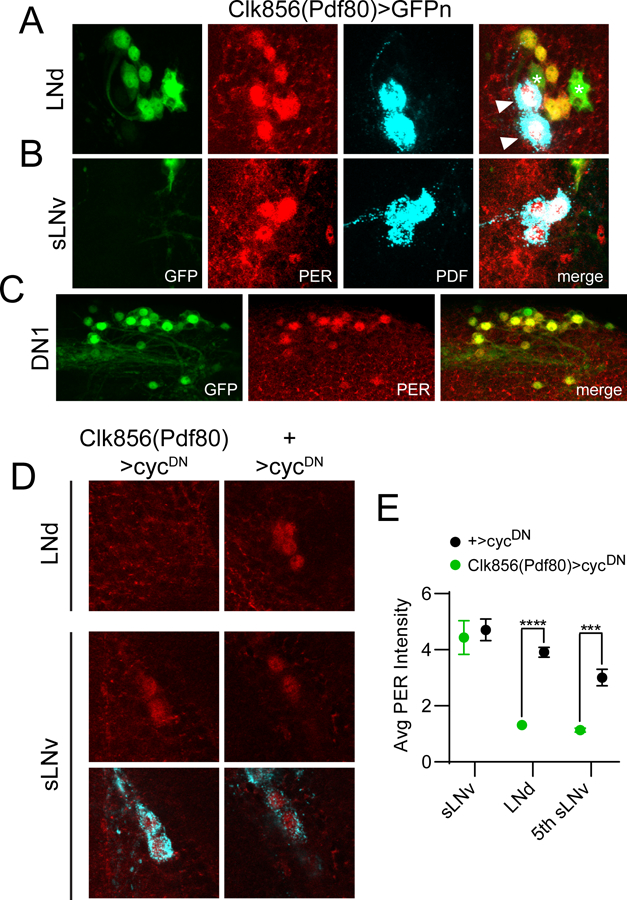
The Clk856(Pdf80) line targets non-sLNv clock cells. For panels A-C, dissections were carried out at ~ZT23 and representative confocal images are shown. GAL4 expression is visualized via a cross to UAS-nGFP (green); PER staining (red) identifies clock cells; PDF staining (cyan) labels LNv cells and processes. A, GFP staining of Clk856(Pdf80)>GFPn brains demonstrating expression in all six LNd cells. In contrast, this line lacks GAL4 expression in PDF+ sLNvs (B) but is present in the vast majority of other non-LNv clock neurons such as DN1 and DN2 cells (C). For A, note that all six PER+ LNds are co-labeled with GFP; however two PDF+ lLNvs (arrowheads) are GFP-. Note also additional expression of this GAL4 line in two PER- non-clock cells (asterisks) near the LNds. D, Representative confocal images are shown of LNd and LNv cells of Clk856(Pdf80)>cycDN flies (left) and +>cycDN control flies (right) stained for PER (red) at ZT23. PDF staining (cyan) was used to identify sLNv cells, and a merged image of PER and PDF staining is shown below each PER staining panel for the sLNvs. E, Quantification of average PER staining intensity at ZT23, normalized to background levels (mean ± SEM), for sLNv cells, LNd cells and the 5th sLNv. n= 8 brains per time point; ***p<0.001; ****p<0.0001, Sidak’s multiple comparisons test following Two-Way ANOVA.
These experiments highlight a role for non-LNv clock neurons in the generation of robust DD rhythms, but they do not indicate whether behavioral rhythmicity depends on broad molecular cycling across multiple clock cell populations or whether specific subsets of non-LNv clock cells make particularly important contributions. To differentiate between these possibilities, we therefore targeted cycDN to subpopulations of non-LNv clock neurons to assess the consequence of molecular clock abrogation. Surprisingly, selective expression of cycDN in populations of LNd, DN1 or DN2 neurons had no effect on rest:activity rhythm strength compared to genetic controls (Table 1; Figure 3C).
Reduction of Rest:Activity Rhythm Strength Following Selective Molecular Clock Abrogation in All Six LNd Neurons
Many clock cell populations are comprised of multiple subgroups that can be divided based on molecular characteristics, including the presence of the photoreceptor cryptochrome (CRY), responsiveness to the neuropeptide Pigment Dispersing Factor (PDF), and selective expression of peptide neurotransmitters (Shafer et al., 2008; Yao and Shafer, 2014; Abruzzi et al., 2017; Chatterjee et al., 2018). For example, there are six LNd neurons, but only three of these express CRY (Picot et al., 2007; Benito et al., 2008; Yoshii et al., 2008). Notably, several GAL4 lines, including those used above for selective expression of cycDN in LNd clock cells (R78G02-GAL4 and MB122B-GAL4), target only the three CRY+ LNds (Schlichting et al., 2016; Guo et al., 2017; Liang et al., 2017). Thus, though we showed that molecular clock cycling in the CRY+ LNds was dispensable for DD rhythms (Figure 3C), it was still possible that clock oscillations in other LNd subsets were necessary or could compensate for the deficit among CRY+ cells. We therefore used DvPdf-GAL4 (Bahn et al., 2009), which in combination with Pdf-GAL80 confers functional GAL4 expression exclusively in four LNd neurons, including the three CRY- cells, and the PDF- 5th sLNv (Guo et al., 2014). We also used a combination of the DvPdf-GAL4 and R78G02-GAL4 lines to drive expression in all six LNds simultaneously. This allowed us for the first time to manipulate the entire LNd population without concurrently affecting other clock cell populations.
We first conducted immunohistochemical analyses to confirm GAL4 expression. When we analyzed R78G02>GFPn brains at ZT23, a time when PER expression should be strong and nuclear, we found that three PER+ LNd neurons were co-labeled with GFP (Figure 5A). We also observed expression in the 5th sLNv (data not shown), which is consistent with previous reports (Schlichting et al., 2016). This GAL4 line is not expressed in other clock cell populations, but it is present in a handful of non-clock cells. Similar analysis with DvPdf-GAL4 combined with Pdf-GAL80 confirmed expression in four PER+ LNds as well as the 5th sLNv. Surprisingly, however, we found substantial GFP expression in the LNvs of DvPdf(Pdf80)>GFPn flies. In fact, even two copies of Pdf-GAL80 were together insufficient to fully suppress DvPdf-GAL4 in LNv neurons (data not shown). We therefore used three copies of Pdf-GAL80 in subsequent experiments conducted with the DvPdf-GAL4 line. These flies retained GAL4-mediated GFPn expression in four LNd cells (Figure 5B) but lacked functional GAL4 in sLNvs (although faint GFPn could still be detected in lLNvs).
Figure 5.
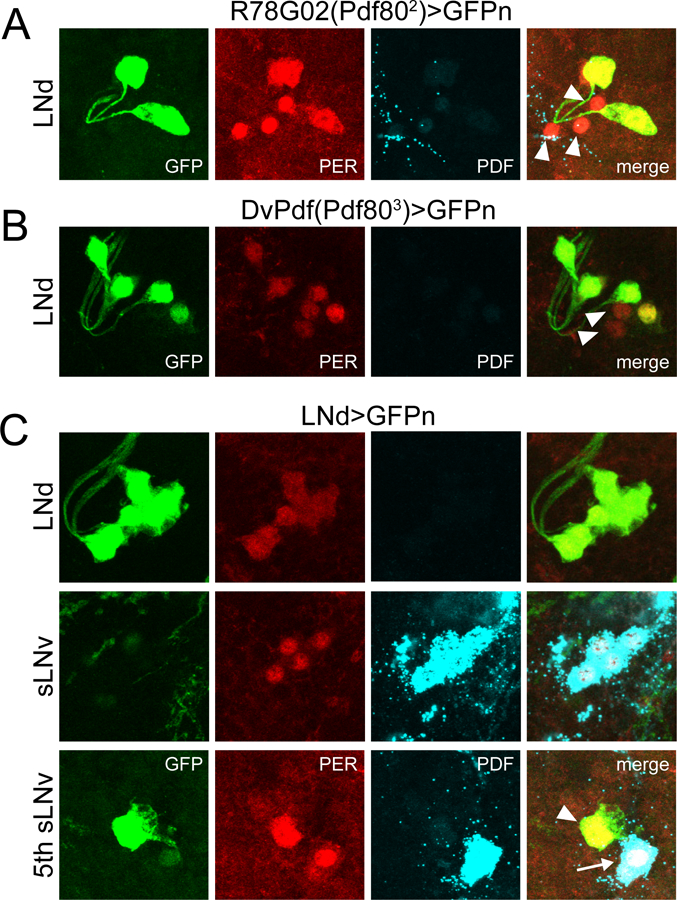
Expression patterns of LNd-targeting GAL4 lines. For all panels, dissections were carried out at ~ZT23 and representative confocal images are shown. GAL4 expression is visualized via a cross to UAS-nGFP (green); PER staining (red) identifies clock cells; PDF staining (cyan) labels LNv cells and processes. A, R78G02(Pdf802)>GFPn brain showing GAL4 expression in a subset of LNd cells. Note the presence of three PER+ LNds that are unlabeled by GFP (arrowheads). B, DvPdf(Pdf803)>GFPn brain showing GAL4 expression in 4 LNd cells. Note that two PER+ LNds are unlabeled by GFP (arrowheads). C, LNd>GFPn brain showing GAL4 expression in all six LNds (top) but lack of expression in PDF+ sLNvs (middle). This line is also strongly expressed in the 5th sLNv (bottom; arrowhead), and has faint residual expression in lLNvs (bottom; arrow).
We also confirmed that the R78G02-GAL4 and DvPdf-GAL4 lines are expressed in complementary populations of LNd neurons, as combining these GAL4 lines along with three copies of Pdf-GAL80 resulted in selective expression in all six PER+ LNds and the 5th sLNv, while avoiding expression in other clock cell populations such as the sLNvs (Figure 5C). We will hereafter refer to this combined GAL4 line as LNd-GAL4. The complete and selective expression of the LNd-GAL4 line in all six LNd cells was corroborated through PER staining of LNd>cycDN brains. This manipulation strongly reduced PER levels and eliminated PER cycling in LNd cells and the 5th sLNv (Figure 6B and C), but it had no effect on the cycling of the PDF+ sLNvs (Figure 6A and C).
Figure 6.
Selective suppression of molecular clock cycling in LNds. A, Representative confocal images of PER staining (red) in sLNv clock cells at different time points taken every six hours on DD1. PDF staining (cyan) was used to identify sLNv cells, and a merged image of PER and PDF staining is shown below each PER staining panel. PER cycling in sLNvs of LNd>cycDN brains (bottom) was indistinguishable from control +>cycDN brains (top). B, Representative confocal images of PER staining (red) in LNd cells taken from the same genotypes and time points as in A. PER levels and cycling are strongly suppressed in LNd neurons of LNd>cycDN flies (bottom) compared to control brains (top), which exhibited normal PER cycling. C, Quantification of average PER staining intensity, normalized to background levels (mean ± SEM), for sLNv cells (top), LNd cells (middle) and the 5th sLNv (bottom). n= 8–9 brains per time point; ****p<0.0001 compared to control PER intensity at that time point, Sidak’s multiple comparisons test following Two-Way ANOVA.
We then assessed the behavioral consequences of suspending the molecular clock in LNd/5th sLNv neurons. In agreement with our previous results, molecular clock abrogation in the CRY+ LNds/5th sLNv had no effect on behavioral rhythms in DD conditions (Table 1; Figure 7A and B). Selective clock suppression in the CRY- LNd cells along with the 5th sLNv with DvPdf(Pdf803)>cycDN similarly failed to alter DD rhythms (Table 1; Figure 7A and B). We did note that use of DvPdf-GAL4 with one or two copies of Pdf-GAL80 to drive cycDN resulted in significantly reduced rhythm strength (Table 1), however we attribute this to the residual LNv expression in these lines. In fact, we found a near complete lack of PDF immunoreactivity in DvPdf(Pdf802)>cycDN flies (data not shown), possibly due to a developmental suppression of CYC leading to a failure of sLNvs to develop properly (Park et al., 2000). In contrast to the lack of effect of clock suppression in individual subsets of LNd neurons, combined manipulation of all six LNd cells along with the 5th sLNv significantly reduced rest:activity rhythm strength in a manner and magnitude similar to that observed when suppressing the clock in all non-LNv cells (Table 1; Figure 7A and B). These data demonstrate that LNd clocks have a necessary role in producing robust rest:activity rhythms in DD conditions.
Neuronal Silencing Reveals a Prominent Role for Non-LNv Clocks in the Generation of DD Rhythms
Thus far, our manipulations eliminated molecular clock cycling in specific clock cell populations but left neuronal communication intact. To determine whether LNv clock neurons are able to drive behavioral rhythms through output circuits that bypass the rest of the clock network, we conducted selective neuronal silencing experiments through GAL4-mediated expression of the Kir2.1 potassium channel (Baines et al., 2001). This manipulation has been shown to strongly hyperpolarize clock cells, rendering them unable to generate action potentials (Wu et al., 2008). As expected, Kir2.1 expression in all clock neurons results in severely blunted DD rest:activity rhythms (Table 1; Figure 8A and B). The reduction in rhythm strength was of a similar magnitude to that observed when molecular cycling was eliminated in the entire clock network (Table 1; Figure 3A). We observed an identical phenotype with drastically decreased rhythm strength when we silenced the LNv clock neurons (Table 1; Figure 8A and B), as has been observed previously (Nitabach et al., 2002; Depetris-Chauvin et al., 2011). Importantly, strong arrhythmicity was also produced by silencing all non-LNv clock neurons in Clk856(Pdf80)>Kir2.1 flies (Table 1; Figure 8A and B). In fact, consistent with previous work (Collins et al., 2012) we found that these flies were as arrhythmic as flies in which the entire clock network was silenced. This represents a much more profound phenotype than that observed following molecular clock suppression in non-LNv clock cells (Table 1; Figure 3A and B). Thus, even though molecular clocks in LNv neurons appear both necessary and sufficient for moderately strong DD rhythms, these LNv cells are unable to drive coherent rhythms in the absence of communication between other nodes of the clock cell network. The difference in phenotypes between arresting the molecular clock and silencing non-LNv clock cells suggests that clock network properties add robustness to the system, wherein functional clocks elsewhere in the network are able to compensate for a lack of molecular cycling in non-LNv cells.
We also directly tested for the consequences of silencing the LNd neurons. Unfortunately, R78G02>Kir2.1 flies did not survive to adulthood, likely due to an effect of Kir2.1 on non-clock neurons in which this line expresses. Nevertheless, we were still able to determine the effect of silencing the CRY+ LNds through use of MB122B-GAL4, which is a split GAL4 line that is exquisitely selective for just four pairs of neurons in the adult brain: the three CRY+ LNds and the 5th sLNv (Guo et al., 2017; Liang et al., 2017). Notably, we found a strong decrease in rhythm strength upon silencing these cells (Table 1; Figure 8C and D). Given the highly selective expression of this GAL4 line, we can unequivocally attribute the reduction in rhythm strength to a suppression of neuronal activity in LNd cells. We also observed a reduction in rhythm strength after silencing the CRY- LNd neurons, although this result was somewhat milder than the effect of silencing the CRY+ subset (Table 1; Figure 8C and D). While both of these LNd manipulations decreased rhythm strength, neither phenotype was as complete as that observed following silencing of all clock cells, as most flies retained some residual rhythmicity. We note, however, that the significant reduction in rhythm strength following electrical silencing of these subsets of LNd neurons stands in contrast to the lack of effect of shutting down the molecular clock in the same cells, again highlighting the fact that clock network properties can compensate for perturbations to molecular cycling in small groups of clock cells. We attempted to combine the MB122B-GAL4 and DvPdf-GAL4 lines in order to assess the consequence of inhibiting neuronal activity in all six LNds, but the resultant flies exhibited weak rhythm strength even in control crosses. Thus, it was impossible to determine whether silencing the entire LNd population further degraded rhythmicity.
Because constitutive neuronal silencing can result in developmental abnormalities (Depetris-Chauvin et al., 2011), it was possible that the behavioral effects of Kir2.1-mediated neuronal silencing reflected improper development of the circadian system rather than an adult-specific contribution of the silenced neuronal populations to rest:activity rhythms. We therefore conducted additional experiments using the TARGET system (McGuire et al., 2004), which allows for temporal control over transgene expression via the inclusion of a temperature-sensitive GAL80 molecule that can inhibit GAL4 at low but not high temperatures. Importantly, we found that flies in which all non-LNv clock neurons were silenced only in adulthood were as arrhythmic as flies in which neuronal silencing was constitutive throughout development. Thus, Clk856(Pdf80)>Kir2.1ts flies were no different from controls when tested at 18°C, when GAL4-mediated transcription was inhibited, but rhythms were strongly compromised at 30°C (Table 1; Figure 9A and B). We also confirmed that adult-specific silencing of MB122B-GAL4-expressing cells reduced rest:activity rhythm strength. Although many of these flies retained fairly strong rhythms, they were clearly reduced in magnitude compared to controls at 30°C but not 18°C (Table 1; Figure 9C and D). This is consistent with the partial reduction in rhythm strength following constitutive silencing of these neurons. In addition to the specific effect of neuronal silencing on rhythm strength, we also noted that temperature independently regulated circadian rhythm strength such that flies typically exhibited stronger rhythms at 30°C than they did at 18°C (Table 1; Figure 9A and C).
To further rule out potential developmental effects, we also assessed whether the reduced rhythmicity associated with adult-specific clock cell silencing could be reversed upon return to 18°C. Though control flies exhibited significant reductions in rest:activity rhythm strength when transferred from 30°C to 18°C (Figure 9F), rhythm strength increased in the vast majority of Clk856(Pdf80)>Kir2.1ts flies (Figure 9E). Interestingly, this recovery took several days (Figure 9G), perhaps due to the kinetics of Kir2.1 turnover after return to the restrictive temperature. We also note that rhythms did not always recover to control levels, which could indicate that extended Kir2.1 expression can induce long-term neuronal dysfunction. Together, these experiments unequivocally demonstrate that acute neuronal silencing is sufficient to drastically compromise rest:activity rhythms.
LNd Neurons Do Not Feed Back Upon sLNv Cells to Regulate Rhythm Strength
The connectivity of the clock network facilitates synchronization of molecular clocks in individual clock cells. For example, release of PDF from LNv neurons regulates the amplitude and period of clock cycling in other clock neurons including the LNds and DN1s (Peng et al., 2003; Lin et al., 2004; Stoleru et al., 2005; Yoshii et al., 2009). Evidence also suggests that DN1 neurons reciprocally regulate LNvs. Thus, DN1-derived glutamate helps maintain coherent molecular cycling in larval LNv neurons (Collins et al., 2014), which are acutely responsive to glutamate application (Hamasaka et al., 2007). In addition, a recent report demonstrated that DN1a neurons can regulate molecular oscillations in LNvs through release of the CCHamide1 peptide (Fujiwara et al., 2018). We therefore conducted experiments to determine whether LNd-specific manipulations that compromise rest:activity rhythm strength do so by altering synchronization of LNv clocks.
We already showed that molecular clock abrogation in LNd neurons (in LNd>cycDN flies) fails to alter PER oscillations in LNv neurons on the first day of DD (Figure 6A and C). However, non-autonomous effects of clock cell manipulations are often subtle and manifest as minor changes in period length of individual clock cells (Lin et al., 2004; Wu et al., 2008; Yoshii et al., 2009). Thus, we assayed PER cycling on the fifth day of DD, which we reasoned would allow subtle alterations to compound over multiple days in free-running conditions. Notably, we found no differences in PER cycling in the sLNvs between LNd>cycDN and control brains, even on the fifth day of DD (Figure 10A and C). In contrast, PER levels were strongly reduced and failed to cycle in LNd neurons and the 5th sLNv under these conditions, confirming the efficacy of the cycDN construct (Figure 10B and C). This suggests that PER cycling in LNvs is not significantly altered by a loss of LNd clocks, although it is possible that very minor effects of LNd clock disruption on sLNv PER cycling may not be appreciable by the fifth day in DD. Nevertheless, it is unlikely that such a minor effect on sLNv PER cycling, if present, could produce the behavioral phenotype of these flies, which is apparent from the initial stages of DD at a time when sLNv PER cycling is unaffected. We conclude that the reduction in rhythm strength following elimination of LNd clocks cannot be accounted for by a subsequent loss of coherence of PER oscillations in PDF+ cells.
We also tested whether electrical silencing of the CRY+ LNd cells in MB122B>Kir2.1 flies affected PER cycling in clock neurons. This manipulation resulted in more prominent reduction in rest:activity rhythm strength compared to suspending LNd clocks. Furthermore, it seemed more likely that any potential feedback from LNd to LNv clock cells would be compromised following neuronal silencing as compared to molecular clock abrogation. Nevertheless, we found no effect of this manipulation on PER cycling in LNv neurons (Figure 11A and C). Interestingly, we also observed normal PER oscillations in LNd neurons in the face of their neuronal silencing (Figure 11B and C). Again, these data argue against a role of LNd neurons in regulating LNv clocks. In conjunction with the fact that silencing of LNv neurons does alter period length of PER oscillations in LNd neurons (Wu et al., 2008), our data support a hierarchical model of the clock network in which, under DD conditions, information flows from sLNv to LNd neurons but not from LNd to sLNv neurons (Stoleru et al., 2005; Chatterjee et al., 2018).
Figure 11.
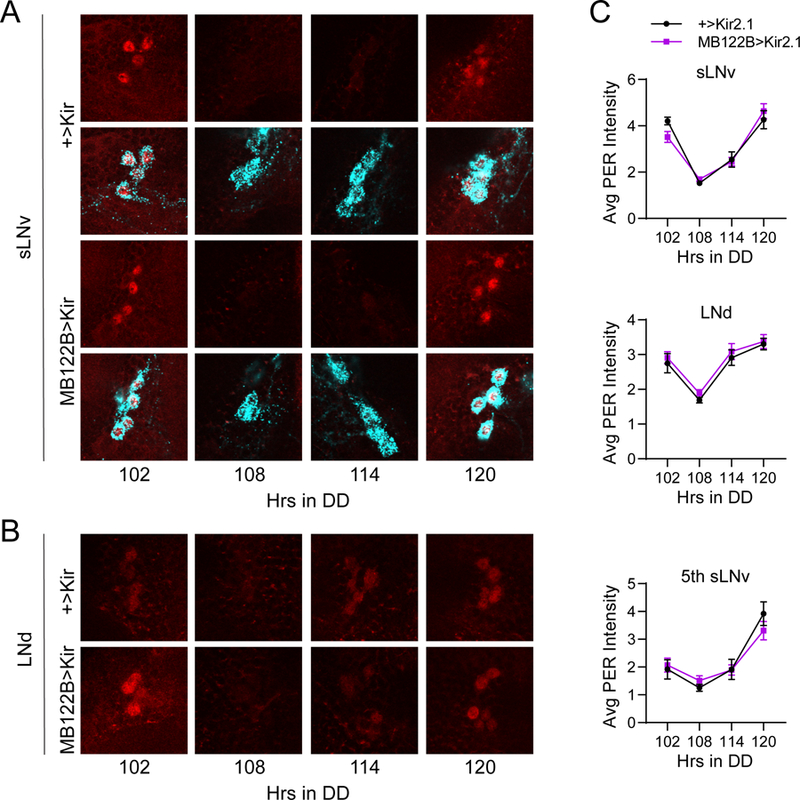
Silencing CRY+ LNds has no effect on LNv clocks. A, Representative confocal images of PER staining (red) in sLNv clock cells at different time points taken every six hours on DD5. PDF staining (cyan) was used to identify sLNv cells, and a merged image of PER and PDF staining is shown below each PER staining panel. PER cycling in sLNvs of MB122B>Kir2.1 brains (bottom) was indistinguishable from control +>Kir2.1 brains (top) even after extended time under free-running conditions. B, Representative confocal images of PER staining (red) in LNd cells taken from the same genotypes and time points as in A. PER levels and cycling are unaffected in LNd neurons of MB122B>Kir2.1 flies (bottom) compared to control brains (top). C, Quantification of average PER staining intensity, normalized to background levels (mean ± SEM), for sLNv cells (top), LNd cells (middle) and the 5th sLNv (bottom). n= 7–9 brains per time point.
Discussion
Though the molecular clock was originally characterized as a cell-autonomous oscillator, it has become increasingly clear that coherent rhythms at the organismal level are an emergent property of the clock cell network and depend critically on communication between clock cells (Dubowy and Sehgal, 2017; Hastings et al., 2018). Because of this, recent research has aimed to identify circuit-level mechanisms through which the information from molecular clocks in individual cells is coordinated among discrete clock cell populations and transmitted across output circuits that directly control physiological and behavioral processes. Importantly, the relatively small number of clock cells in the fly enables manipulations of these neurons individually or in small groups in order to assess their relative contributions to circadian behavior (Venken et al., 2011). This has made Drosophila a powerful model system for delineating the output pathways by which consolidated signals from various clock cell populations generate meaningful behavior.
To help understand the organizational logic of the circadian system, we conducted experiments that either eliminated molecular clock function or suppressed neuronal firing in discrete groups of clock cells. We largely reaffirmed the necessity of sLNv neurons for behavioral rhythmicity in conditions (Helfrich-Forster, 1998; Renn et al., 1999; Stoleru et al., 2004) and likewise confirmed that loss of molecular clock function in sLNvs drastically reduces rest:activity rhythm strength (Stoleru et al., 2004; Tanoue et al., 2004; Picot et al., 2007; Rieger et al., 2009). The reduction in rhythmicity achieved by these manipulations likely arises from the loss of the normal function of sLNv neurons to coordinate molecular oscillations (Peng et al., 2003; Lin et al., 2004; Yoshii et al., 2009) and to properly phase neuronal activity rhythms (Liang et al., 2016, 2017) in other groups of clock cells. This latter role may be particularly important given that the loss of PDF signaling shifts the phase of neuronal activity rhythms in different clock cell populations by several hours, even under LD conditions, while the effect on PER cycling is only appreciable after several days in DD.
The sLNvs have also been described as being sufficient for DD rhythms because restoration of the per gene specifically to PDF+ LNvs was shown to largely rescue rest:activity rhythm strength (Grima et al., 2004; Picot et al., 2007). Our findings offer a more nuanced understanding of sLNv function. Though flies with clocks only in LNv neurons retain behavioral rhythms in DD, the resultant rhythms are of substantially reduced strength. This points to an essential role for non-LNv clocks in the production of normal DD rest:activity rhythms. The consequences of silencing non-LNv clock cells are even more severe, definitively demonstrating that sLNvs are not sufficient for behavioral rhythms. These findings are consistent with the idea that circadian rhythmicity results from the combined contribution of multiple interdependent clock cell populations (Yao and Shafer, 2014; Beckwith and Ceriani, 2015) and that coherent behavioral rhythms in Drosophila require largescale network synchrony (Yao et al., 2016). Importantly, the more profound effect of electrical silencing compared to molecular clock suppression also demonstrates that the circadian network confers robustness against perturbations of the molecular clock. One possibility to explain this result is that communication with other clock neurons maintains rhythms of electrical firing (Liang et al., 2016) such that molecular cycling is not absolutely required.
In this respect, the fly circadian network appears to share functional similarities with that of mammals. Thus, in the suprachiasmatic nucleus (SCN), the master circadian clock in mammals, an intact clock network can maintain molecular cycling in the face of mutations to core clock genes, demonstrating that intracellular communication allows for resilience of circadian clock function (Liu et al, 2007). Furthermore, intercellular communication in the SCN is necessary for coherent molecular rhythms between individual SCN neurons (Yamaguchi et al., 2003). More recent evidence suggests that SCN communication is also necessary for behavioral rhythms. Thus, synaptic inhibition of SCN neurons that produce the NMS peptide, which is a broadly expressed marker that includes multiple peptidergic subtypes of SCN cells, eliminates behavioral rhythms in mice (Lee et al., 2015). Whether more directed silencing of smaller populations of SCN clock cells also degrades behavioral rhythms in mammals is unknown, but our results in flies suggest that this may indeed be the case.
In addition to investigating the role of non-LNv neurons broadly, we also conducted more directed experiments to assess the contribution of subsets of non-LNv clock cells to DD rhythms. We focused particularly on the LNd/5th sLNv neurons for a few reasons. First, these cells have a prominent role under LD conditions in producing the evening peak of activity that occurs in anticipation of lights-off (Grima et al., 2004). Second, they are capable of functioning as master oscillators in constant light (Picot et al., 2007; Rieger et al., 2009). Finally, it was unclear to what extent the LNds/5th sLNv contribute to DD rhythms. Although initial ablation studies concluded that these cells are dispensable (Stoleru et al., 2004), a more recent report argued for a dominant role of the LNds/5th sLNv in controlling locomotor outputs of the clock network by demonstrating that inhibition of electrical activity or synaptic signaling in cells labeled by the DvPdf-GAL4 line induced strong arrhythmicity in both LD and DD conditions (Guo et al., 2014).
To effectively probe the role of a group of neurons, a GAL4 driver must be both comprehensive (i.e. label all cells within a population) and specific (i.e. label only the cells within a population). To our knowledge, no existing GAL4 line satisfies these two criteria with respect to the LNds/5th sLNv. Though some lines like the split MB122B-GAL4 offer exquisite specificity, they label only a subset of LNds. This lack of comprehensiveness could be problematic if the population is able to compensate for a manipulation in a proportion of its cells via the influence of the remaining non-manipulated subset, such as is the case for disco mutants in which even a single surviving sLNv is sufficient to produce rhythmic behavior (Helfrich-Forster, 1998). We indeed found this to be true for the CRY+ and CRY- LNd neurons. Although elimination of clock cycling via MB122B-GAL4, R78G02-GAL4 or DvPdf-GAL4(Pdf-GAL803) was without effect, rest:activity rhythm strength was significantly reduced when we eliminated molecular clock function with a novel combination of GAL4 lines that targeted the entire subset of LNds with an unprecedented mix of comprehensiveness and selectivity. Our results therefore demonstrate an important contribution of molecular cycling in LNd/5th sLNv neurons to the production of strong DD rhythms.
Interestingly, as was the case for the non-LNv population as a whole, electrical silencing of the LNds/5th sLNv evoked stronger phenotypes than did elimination of molecular clock function. Thus, Kir2.1-mediated inhibition of either the CRY+ or CRY- subsets of LNds substantially reduced rhythm strength despite a lack of effect of selectively eliminating molecular clock function in these cells. Notably, we confirmed the reduction in DD rhythm strength produced by silencing DvPdf-GAL4+ cells but found the effect to be much subtler than reported previously (Guo et al., 2014). One potential explanation for this discrepancy is that we used multiple copies of Pdf-GAL80 to definitively prohibit GAL4 function in LNv cells, as immunohistochemical analysis demonstrated that a single copy of Pdf-GAL80, as was used by Guo et al., did not completely prevent GAL4-mediated transcription in these neurons.
Together, our results demonstrate that, while molecular cycling of the LNds may be redundant among the population, at least one LNd must retain its endogenous rhythm and a majority (if not all) LNds must be able to transmit information in order to maintain strong behavioral rhythmicity in DD conditions. This establishes the LNds as necessary to serve two functions in DD: first, to provide at least some rhythmic information derived from their endogenous cycling; and second, to communicate with as-yet-unidentified parallel or downstream targets. Unfortunately, we were unable to assess the effect of silencing all six LNd neurons concurrently, so future work will focus on the creation of additional GAL4 lines that mark the LNds completely and exclusively. These lines will help determine whether LNd neuronal activity is absolutely essential for DD rhythms or whether circadian information can bypass this population such that some degree of rhythmicity can be retained despite their inhibition. Furthermore, as all of our behavioral testing was conducted in DD conditions, it will be of interest to assess the consequences of LNd manipulations on behavioral rhythmicity in LD and LL conditions. Though previous work has strongly implicated subsets of these neurons in regulating locomotor rhythms under such conditions (Grima et al., 2004; Stoleru et al., 2004; Picot et al., 2007; Rieger et al., 2009), it is possible that even more robust phenotypes will be observed through simultaneous manipulation of the entire LNd population.
Our results have important implications for our understanding of the neuronal circuits through which clock cells modulate locomotor activity. The inability of PDF+ cells to independently drive behavioral rhythms in the face of electrically silencing non-LNv cells could be due to the necessity of feedback input from other groups of clock neurons to maintain coherent molecular rhythms in LNvs, as has been suggested for DN1 cells (Collins et al., 2012, 2014). Alternatively, it could be because non-LNv clock neurons serve as necessary conduits between LNvs neurons and downstream output cells. Though our manipulations of LNd cells do not rule out the former possibility, they do demonstrate that DD behavioral rhythms can be strongly compromised in the absence of obvious deficits in the molecular cycling of LNv neurons. This argues against the possibility that PDF+ cells can independently modulate locomotor outputs through pathways that bypass the rest of the clock network, and furthermore suggests that the LNds directly regulate DD rest:activity rhythms in a previously underappreciated manner.
These findings are in line with a number of previous studies that have demonstrated that the LNvs are not alone sufficient for normal activity rhythms, nor are they absolutely necessary for some residual rhythmicity to be present. In fact, early studies using genetic mosaic or transgenic approaches to restore per expression to small subsets of neurons in otherwise per mutant animals designated the lateral neurons as a whole (including both LNvs and LNds) as master pacemakers (Ewer et al., 1992; Frisch et al., 1994). Subsequent studies that specifically tested the contribution of the LNvs emphasized their importance for DD rhythmicity but nonetheless could not rule out a role for other non-LNv subsets. For example, an appreciable percentage of flies in which PDF+ cells have been ablated retain behavioral rhythms in DD, although with greatly reduced power compared to controls (Renn et al., 1999). Further evidence that sLNvs are not absolutely necessary for DD rhythms comes from disco mutants. Many of these flies lack all lateral neurons and are completely arrhythmic under conditions of constant darkness (Dushay et al., 1989); however, ~20% of disco mutants exhibit some rhythmicity, and the vast majority of these have no LNvs present (Helfrich-Forster, 1998). This rhythmicity is unlikely due to dorsal clock neurons, which are present and maintain PER oscillations in all disco mutants (Blanchardon et al., 2001), and instead could be due to the occasional intact LNd cell, as has been suggested (Helfrich-Forster, 1998). The contribution of LNd neurons in addition to LNvs to DD rhythms is also demonstrated through per rescue experiments. Thus, restoration of per to both LNv neurons and the CRY+ LNds/5th sLNv results in stronger, more coherent rhythms than restoration to LNvs alone (Grima et al., 2004). Similarly, it was shown using manipulations that cell-autonomously speed up or slow down the molecular clock that flies in which clocks are coordinately manipulated in both LNd and LNv subsets have more coherent free-running rhythms than flies in which the LNv subset is manipulated alone (Yao et al., 2016). Taken together with our results, these experiments demonstrate that non-LNv clock cells, and in particular LNd neurons, are important regulators of DD rhythms.
Though we have shown that LNd cells make essential contributions to DD rhythmicity, our data, and that of others, demonstrates a more profound effect of LNv manipulations under such conditions. For example, we found that flies lacking LNv clocks are less rhythmic than flies lacking LNd clocks. Furthermore, in contrast to the inability of LNd manipulations to alter molecular clock cycling in LNvs, manipulations of LNv neurons produce changes in molecular clocks within other neurons of the clock network, including at least some LNds (Stoleru et al., 2005; Wu et al., 2008; Yao and Shafer, 2014). These data support a semi-hierarchical model in which LNd neurons, and likely other clock cell populations, lie downstream of the LNvs in a circuit controlling DD rhythmicity (Chatterjee et al., 2018). It is also possible that information from the sLNvs and LNds/5th sLNv independently converges on a downstream output node within the clock network, provided neither sLNv nor LNd-derived information is sufficient to produce normal output on its own. Future experiments aimed at delineating the anatomical and functional connectivity between the nodes of the clock network and identified non-clock output cell populations (Cavanaugh et al., 2014; Cavey et al., 2016) should further clarify the organizational logic of Drosophila clock network output.
Acknowledgements:
We thank Thomas Clandinin, Fumika Hamada, Michael Rosbash, Amita Sehgal, and Orie Shafer for fly stocks and Amita Sehgal for antisera.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
References
- Abruzzi KC, Zadina A, Luo W, Wiyanto E, Rahman R, Guo F, Shafer O, Rosbash M (2017) RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLOS Genet 13:e1006613 Available at: http://dx.plos.org/10.1371/journal.pgen.1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn JH, Lee G, Park JH (2009) Comparative analysis of Pdf-mediated circadian behaviors between Drosophila melanogaster and D. virilis. Genetics 181:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M (2001) Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 21:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith EJ, Ceriani MF (2015) Experimental assessment of the network properties of the Drosophila circadian clock. J Comp Neurol 523:982–996. [DOI] [PubMed] [Google Scholar]
- Benito J, Houl JH, Roman GW, Hardin PE (2008) The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms 23:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchardon E, Grima B, Klarsfeld A, Chelot E, Hardin PE, Preat T, Rouyer F (2001) Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci 13:871–888. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Geratowski JD, Wooltorton JRA, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A (2014) Identification of a circadian output circuit for rest: Activity rhythms in drosophila. Cell 157:689–701 Available at: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Collins B, Bertet C, Blau J (2016) Circadian rhythms in neuronal activity propagate through output circuits. Nat Neurosci 19:587–595 Available at: http://www.nature.com/doifinder/10.1038/nn.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Lamaze A, De J, Mena W, Chélot E, Martin B, Hardin P, Kadener S, Emery P, Rouyer F (2018) Reconfiguration of a Multi-oscillator Network by Light in the Drosophila Circadian Clock. Curr Biol:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Kane EA, Reeves DC, Akabas MH, Blau J (2012) Balance of Activity between LNvs and Glutamatergic Dorsal Clock Neurons Promotes Robust Circadian Rhythms in Drosophila. Neuron 74:706–718 Available at: 10.1016/j.neuron.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Kaplan HS, Cavey M, Lelito KR, Bahle AH, Zhu Z, Macara AM, Roman G, Shafer OT, Blau J (2014) Differentially Timed Extracellular Signals Synchronize Pacemaker Neuron Clocks. PLoS Biol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BR, Young MW (2014) Interactive Features of Proteins Composing Eukaryotic Circadian Clocks. Annu Rev Biochem 83:191–219 Available at: http://www.annualreviews.org/doi/10.1146/annurev-biochem-060713-035644. [DOI] [PubMed] [Google Scholar]
- Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF (2011) Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol 21:1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowy C, Sehgal A (2017) Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics 205:1373–1397 Available at: http://proxy.library.upenn.edu:4742/content/205/4/1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay MS, Rosbash M, Hall JC (1989) The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J Biol Rhythms 4:1–27. [DOI] [PubMed] [Google Scholar]
- Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC (1992) Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells’ influence on circadian behavioral rhythms. J Neurosci 12:3321–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch B, Hardin PE, Hamblen-Coyle MJ, Rosbash M, Hall JC (1994) A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron 12:555–570. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Hermann-Luibl C, Katsura M, Sekiguchi M, Ida T, Helfrich-Forster C, Yoshii T (2018) The CCHamide1 Neuropeptide Expressed in the Anterior Dorsal Neuron 1 Conveys a Circadian Signal to the Ventral Lateral Neurons in Drosophila melanogaster. Front Physiol 9:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin C-C, Potter CJ, Clandinin TR (2011) A versatile in vivo system for directed dissection of gene expression patterns. Nat Methods 8:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Ché Lot E, Xia R, Rouyer FO (2004) Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431:869–873. [DOI] [PubMed] [Google Scholar]
- Gummadova JO, Coutts GA, Glossop NRJ (2009) Analysis of the Drosophila Clock promoter reveals heterogeneity in expression between subgroups of central oscillator cells and identifies a novel enhancer region. J Biol Rhythms 24:353–367. [DOI] [PubMed] [Google Scholar]
- Guo F, Cerullo I, Chen X, Rosbash M (2014) PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. Elife 3:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Chen X, Rosbash M (2017) Temporal calcium profiling of specific circadian neurons in freely moving flies. Proc Natl Acad Sci U S A 114:E8780–E8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaka Y, Rieger D, Parmentier M-L, Grau Y, Helfrich-Forster C, Nassel DR (2007) Glutamate and its metabotropic receptor in Drosophila clock neuron circuits. J Comp Neurol 505:32–45. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M (2018) Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19:453–469. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C (1998) Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol A 182:435–453. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Head LM, Ling J, Tang X, Liu Y, Hardin PE, Emery P, Hamada FN (2012) Circadian rhythm of temperature preference and its neural control in Drosophila. Curr Biol 22:1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, Takahashi JS, Yanagisawa M (2015) Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron 85:1086–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Holy TE, Taghert PH (2016) Synchronous Drosophila circadian pacemakers display nonsynchronous Ca2+ rhythms in vivo. Science (80- ) 351:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Holy TE, Taghert PH (2017) A Series of Suppressive Signals within the Drosophila Circadian Neural Circuit Generates Sequential Daily Outputs. Neuron 94:1173–1189.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH (2004) The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24:7951–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL (2004) Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004:pl6. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS (2012) Central and Peripheral Circadian Clocks in Mammals. Annu Rev Neurosci 35:445–462 Available at: http://www.annualreviews.org/doi/10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC (2002) Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109:485–495. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A 97:3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M (2003) Drosophila free-running rhythms require intercellular communication. PLoS Biol 1:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F (2007) Light Activates Output from Evening Neurons and Inhibits Output from Morning Neurons in the Drosophila Circadian Clock. PLOS Biol 5:e315 Available at: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC., Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell 99:791–802 Available at: http://linkinghub.elsevier.com/retrieve/pii/S0092867400816761. [DOI] [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K, Helfrich-Forster C (2006) Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 26:2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Wülbeck C, Rouyer F, Helfrich-Förster C (2009) Period Gene Expression in Four Neurons Is Sufficient for Rhythmic Activity of Drosophila melanogaster under Dim Light Conditions. J Biol Rhythms 24:271–282 Available at: http://journals.sagepub.com/doi/10.1177/0748730409338508. [DOI] [PubMed] [Google Scholar]
- Ryder E et al. (2004) The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167:797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M, Menegazzi P, Lelito KR, Yao Z, Buhl E, Dalla Benetta E, Bahle A, Denike J, Hodge JJ, Helfrich-Forster C, Shafer OT (2016) A Neural Network Underlying Circadian Entrainment and Photoperiodic Adjustment of Sleep and Activity in Drosophila. J Neurosci 36:9084–9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH (2008) Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R (2003) Genetic analysis of the circadian system in Drosophila melanogaster and mammals. J Neurobiol 54:111–147. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernández M de la P, Menet JS, Ceriani MF, Rosbash M (2007) The Drosophila circadian network Is a seasonal timer. Cell 129:207–219. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M (2004) Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431:862–868. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M (2005) A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438:238–242. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE (2004) Circadian Clocks in Antennal Neurons Are Necessary and Sufficient for Olfaction Rhythms in Drosophila. Curr Biol 14:638–649 Available at: http://www.sciencedirect.com/science/article/pii/S0960982204002611. [DOI] [PubMed] [Google Scholar]
- Veleri S, Brandes C, Helfrich-Forster C, Hall JC, Stanewsky R (2003) A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol 13:1758–1767. [DOI] [PubMed] [Google Scholar]
- Venken KJT, Simpson JH, Bellen HJ (2011) Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72:202–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Young MW (1995) Circadian rhythms in Drosophila can be driven by period expression in a restricted group of central brain cells. Neuron 15:345–360. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cao G, Nitabach MN (2008) Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms 23:117–128. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H (2003) Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302:1408–1412. [DOI] [PubMed] [Google Scholar]
- Yao Z, Bennett AJ, Clem JL, Shafer OT (2016) The Drosophila Clock Neuron Network Features Diverse Coupling Modes and Requires Network-wide Coherence for Robust Circadian Rhythms. Cell Rep 17:2873–2881 Available at: 10.1016/j.celrep.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Shafer OT (2014) The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science (80- ) 343:1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C (2008) Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol 508:952–966. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Forster C (2009) The Neuropeptide Pigment-Dispersing Factor Adjusts Period and Phase of Drosophila’s Clock. J Neurosci 29:2597–2610 Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R (2010a) DN1p Circadian Neurons Coordinate Acute Light and PDF Inputs to Produce Robust Daily Behavior in Drosophila. Curr Biol 20:591–599 Available at: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P (2010b) Light and Temperature Control the Contribution of Specific DN1 Neurons to Drosophila Circadian Behavior. Curr Biol 20:600–605 Available at: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


