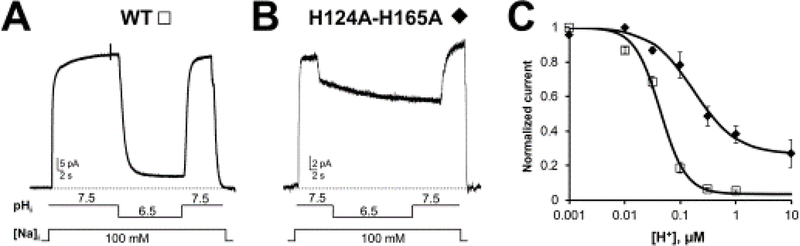
Figure 3: Two histidines are important for NCX pH regulation.
Exchanger currents were recorded using the giant excised patch technique in the inside out configuration from Xenopus laevis oocytes expressing the indicated constructs (modified from [9]). A. The WT cardiac exchanger is highly sensitive to changes in intracellular pH and its activity is almost completely abolished in the presence of cytoplasmic pH 6.5. B. Mutation of histidine 124 and 165 to alanine drastically decreased the exchanger pH sensitivity, underlying the role of these two residues in the regulation of NCX by protons. C. Dose response curves for cytoplasmic H+ for WT (cardiac isoform NCX1.1) and H124A-H165A mutant exchangers. Replacement of histidine 124 and 165 drastically decreased the sensitivity of NCX to cytoplasmic protons and significantly changed the cooperativity of binding.