Figure 1.
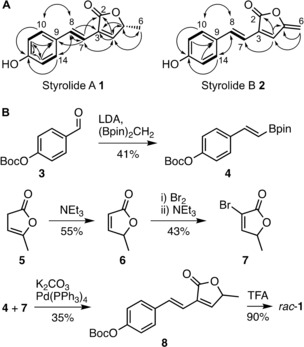
A) Chemical structures of styrolide A (1) and styrolide B (2) with key correlations observed by 2D NMR spectroscopy. Bold lines: 1H‐1H COSY correlations; solid arrows: HMBC correlations. B) Total synthesis of styrolide A (1). Enantiomers of rac‐1 were separated by HPLC on a chiral stationary phase. Boc=tert‐butoxycarbonyl, LDA=lithium diisopropyl amide, (Bpin)2CH2=bis[(pinacolato)boryl]methane, TFA=trifluoroacetic acid.
