Figure 1.
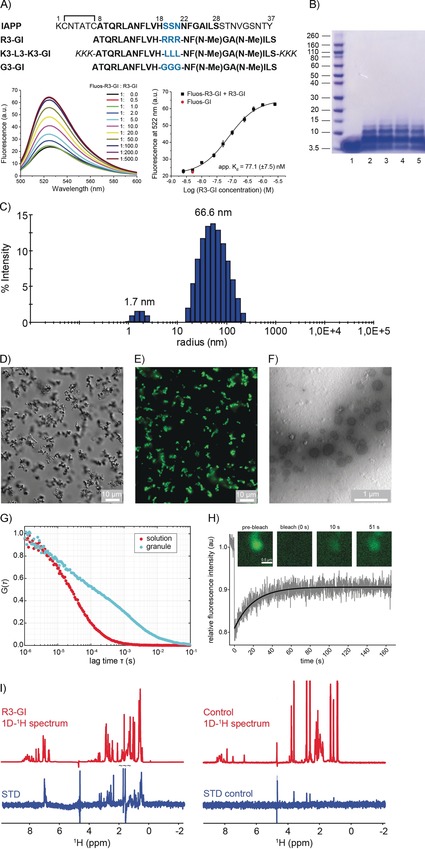
R3‐GI self‐assembly. A) Top: sequences and abbreviations of IAPP and the investigated ISMs. “Hot segments” of the IAPP interaction surface with Aβ40 are shown with black bold letters; residues in the IAPP loop and ISM linker regions are drawn in blue. The L3‐GI solubilizing tag “KKK” is shown in italics. Bottom: fluorescence emission spectra of N‐terminal fluorescein‐labeled R3‐GI (Fluos‐R3‐GI; 5 nm) alone and after titration with R3‐GI at the indicated molar ratios (pH 7.4). On the right, the binding curve is shown. The estimated app. K d value for R3‐GI self‐association is 77.1(±7.5) nm. Data are means (±SEM) from three binding curves. B) R3‐GI oligomerization (500 μm) studied by cross‐linking with glutaraldehyde and NuPAGE. Lane 1: R3‐GI without cross‐linking; lanes 2–5: R3‐GI cross‐linked following incubation for 0 h (lane 2), 1 h (lane 3), 24 h (lane 4), and 48 h (lane 5). C) DLS studies of a 50 μm R3‐GI in 10 mm sodium phosphate buffer, pH 7.4 (containing 1 % v/v HFIP). D) Differential interference contrast (DIC) micrograph of R3‐GI (500 μm). The peptide was imaged immediately after dissolution into 10 mm sodium phosphate buffer, pH 7.4 (containing 1 % v/v HFIP) without filtration. Scale bar: 10 μm. E) Microscopy image of Fluos‐R3‐GI in fluorescence mode. F) TEM image of R3‐GI (500 μm) in 10 mm sodium phosphate buffer, pH 7.4 (containing 1 % v/v HFIP). Spherical assemblies were observed with diameters in the range of 200–400 nm. G) Fluorescence correlation spectroscopy (FCS) of a mixture of 454 μm R3‐GI and 454 nm Fluos‐R3‐GI showing that the translational diffusion inside the R3‐GI granules is strongly retarded. H) Fluorescence recovery after photobleaching (FRAP) of a R3‐GI granule. The fast recovery of the fluorescence intensity indicates that the majority of the peptide molecules are exchanging with the bulk solution within seconds. I) Saturation transfer difference (STD) NMR experiments of 1.25 mm R3‐GI in 10 mm sodium phosphate buffer, pH 7.4 (containing 1 % v/v HFIP and 10 % v/v D2O), showing that soluble, monomeric R3‐GI undergoes exchange with a high‐molecular‐weight oligomeric R3‐GI conformer. As a control, STD spectra of a 60 μm solution of the peptide GVAEPEQDCAVTSGE (Mr=1.492 kDa) are shown. The control peptide is monomeric under the conditions employed in the experiment.
