Table 1.
Comparison of the aldehyde substrate scope of OXCMe and HACLHs.[a]
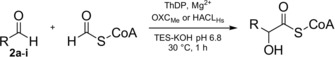
|
Aldehyde |
R |
Product name |
OXCMe [c] |
HACLHs [c] |
|---|---|---|---|---|
|
2 a |
H |
glycolyl‐CoA |
100 |
11 |
|
2 b |
Me |
lactyl‐CoA |
74 |
100 |
|
2 c |
CH2Me |
2‐hydroxybutyryl‐CoA |
5 |
100 |
|
2 d |
CH2OH |
glyceryl‐CoA |
1 |
100 |
|
2 e |
CHOHCH2OH |
erythronyl‐CoA |
n.d.[b] |
n.d.[b] |
|
2 f |
COOH |
tartronyl‐CoA |
n.d.[b] |
n.d.[b] |
|
2 g |
(CH2)2COOH |
2‐hydroxyglutaryl‐CoA |
1 |
100 |
|
2 h |
Ph |
mandelyl‐CoA |
100 |
3 |
|
2 i |
CH2Ph |
3‐phenyllactyl‐CoA |
22 |
100 |
[a] The reaction contained 2 a–2 i (10 mm), formyl‐CoA (1 mm), OXCMe or HACLHs (5 μm). Products were analyzed by LC‐MS after 1 h reaction time. [b] Product not detected. [c] Relative activity in %. Relative activity refers to the comparison of OXCMe and HACLHs for each aldehyde substrate.
