Scheme 4.
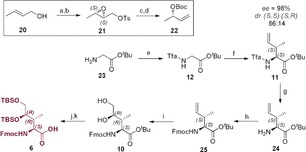
Synthesis of side chain‐protected Fmoc‐(3R,4R)‐4,5‐l‐dihydroxyisoleucine (6) a) (+)‐DIPT, Ti(OiPr)4, TBHP, DCM, −20 °C, 4 h; b) TsCl, Et3N, DMAP, DCM, −10 °C, 30 h, 67 %; c) NaI, Zn(Cu), THF, 70 °C, 2 h; d) Boc2O, NaH, THF, 0 °C to r.t., 16 h, 75 %; e) ethyl trifluoroacetate, NEt3, MeOH, r.t., 16 h, quant.; f) LHMDS, ZnCl2, PPh3, [(p‐cymene)RuCl2]2, 22, THF, −72 °C to r.t., 16 h, 88 %; g) NaBH4, MeOH, r.t., 1 h; h) FmocOSu, Et3N, dioxane, r.t., 4 h, 82 %; i) K2OsO4⋅H2O, NMO, CHCl3/H2O, r.t., 6 h, 40 %; j) TBSCl, pyridine/DMF(1:9), r.t., 24 h, 95 %; k) TMSOTf, 2,6‐lutidine, 0 °C to r.t., 2 h, 90 %.
