Summary
In this nationwide, population‐based study, we assessed trends in primary treatment and survival among 687 patients with nodular lymphocyte‐predominant Hodgkin lymphoma (75% males; median age, 40 years; and 74% stage‐I/II disease) diagnosed in the Netherlands between 1993–2016. There were no noteworthy changes in the application of primary therapy over time among adult patients across the different disease stages and age groups. Survival among various subgroups of adult patients was largely comparable to the expected survival of the general population. A particularly encouraging finding was that young adult patients experienced virtually no excess mortality, as compared to the general population.
Keywords: nodular lymphocyte‐predominant Hodgkin lymphoma (NLPHL), cancer epidemiology, population‐based registry, incidence, survival
Nodular lymphocyte‐predominant Hodgkin lymphoma (NLPHL) is a rare, distinct subtype of Hodgkin lymphoma (HL). It differs substantially from classical HL regarding clinical, immunological, and histological characteristics (Anagnostopoulos et al., 2000). Also, the management of NLPHL varies from classic HL, especially for patients with limited‐stage NLPHL in whom radiotherapy is generally indicated (Eichenauer et al., 2018; Spinner et al., 2019). Furthermore, the universal expression of CD20 in NLPHL led to the incorporation of rituximab into treatment algorithms around 2003 (Eichenauer et al., 2011).
Evidence to guide treatment decision‐making in NLPHL has come from the few prospective and retrospective studies performed over the past decades (Chen et al., 2010; Alonso et al., 2018). Therefore, it is reasonable to assume that NLPHL management might vary across time and geography. In this regard, population‐based studies can provide insights on how treatment practices over time affect the population‐level survival of patients. For example, the few published population‐based studies in NLPHL provide conflicting results about the effect of rituximab on survival (Molin et al., 2017; Shivarov & Ivanova, 2018). These studies, however, have their limitations, including small sample sizes, lack of detailed treatment information, or a short length of follow‐up.
Given the scarcity of clinical and population‐based studies in NLPHL, we conducted a large, contemporary, nationwide population‐based study to assess trends in primary treatment and survival among NLPHL patients diagnosed during a 24‐year period in the Netherlands.
Methods
We selected all 687 NLPHL patients diagnosed between 1993 and 2016 — with survival follow‐up through February 2018 — from the nationwide population‐based Netherlands Cancer Registry (NCR) using the International Classification of Diseases for Oncology morphology code 9659 that was introduced in 1993. Established in 1989, the NCR has a coverage of >95% of all newly diagnosed malignancies in the Netherlands (Schouten et al., 1993). Information on dates of birth and diagnosis, sex, stage, vital status (i.e. alive, dead, or emigration) and primary therapy started within one year after diagnosis (i.e. no anti‐neoplastic therapy — including active surveillance and lymph node excision — radiotherapy alone, or treatment with a chemotherapeutic backbone) was available in the NCR. Data on the application of rituximab and the exact therapeutic regimen were registered in the NCR for patients diagnosed from 2007 and 2014 respectively (Dinmohamed et al., 2017). The Privacy Review Board of the NCR approved the use of anonymous data for this study.
Relative survival (RS) was calculated to estimate disease‐specific survival according to the cohort method (Dickman & Adami, 2006). RS is defined as the ratio of the overall survival (OS) of patients to the expected survival (ES) of an equivalent group from the general population, matched to the patients by age, sex and time period. ES was estimated from Dutch population life tables using the Ederer II method (Ederer & Heise, 1959). RS was calculated up to ten years after diagnosis according to calendar period (1993–2002 and 2003–2016), sex, age (18–39, 40–59, and ≥60 years), and stage (I/II and III/IV). The first and last calendar periods represent the pre‐ and post‐rituximab era, respectively. Multivariable evaluation of RS was performed using Poisson regression to assess the relative excess risk of mortality within ten years after diagnosis (Dickman et al., 2004). Also, OS was calculated up to ten years after diagnosis to present outcomes according to primary therapy stratified by stage. P < 0·05 indicated statistical significance. Seventy‐one patients <18 years at diagnosis were only included for incidence analysis. Further details about the statistical analyses are provided in the Data S1.
Results
Baseline patient characteristics are presented in Table SI. Most patients were male (75%) and aged ≥40 years (51%), and had stage‐I/II disease (74%) and no B symptoms (70%). Females were older than males (45 years vs. 39 years; P = 0·015). The overall age‐standardized incidence rate increased from 1·0/1 000 000 to 2·2/1 000 000 person‐years between 1993–2002 and 2003–2016 respectively. This was a result of the increasing incidence among adults (Figure S1). Moreover, the median age increased from 34 to 42 years between 1993–2002 and 2003–2016 respectively (P < 0·001). NLPHL mainly affects young and middle‐aged adults (Figure S2).
There were no noteworthy trends in primary therapy over time among adult patients across the different disease stages and age groups (data not shown). Overall, radiotherapy alone was the treatment of choice in limited‐stage disease (i.e. stage I/II), whereas therapy with a chemotherapeutic backbone was preferred in advanced‐stage disease (i.e. stage III/IV; Fig 1A and Table SII). This was independent of age. Of note, patients with stage‐I disease more often received radiotherapy alone than those with stage II disease (62% vs. 46%; P < 0·001). Chemotherapy was combined with radiotherapy among 45% and 9% of the chemotherapy recipients with limited‐ and advanced‐stage disease, respectively (P < 0·001). During 2007–2016, 40% and 57% of the chemotherapy recipients with limited‐ and advanced‐stage disease received chemotherapy with rituximab (P = 0·095). The application of rituximab remained comparatively steady during this period. Lastly, rituximab monotherapy was rarely applied. Detailed data of 143 adult patients diagnosed during 2014–2016 (median age, 50 years; age range, 18–81 years) revealed that 84% of the chemotherapy recipients were treated with ABVD [Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine; Fig 1B and Table SIII].
Figure 1.
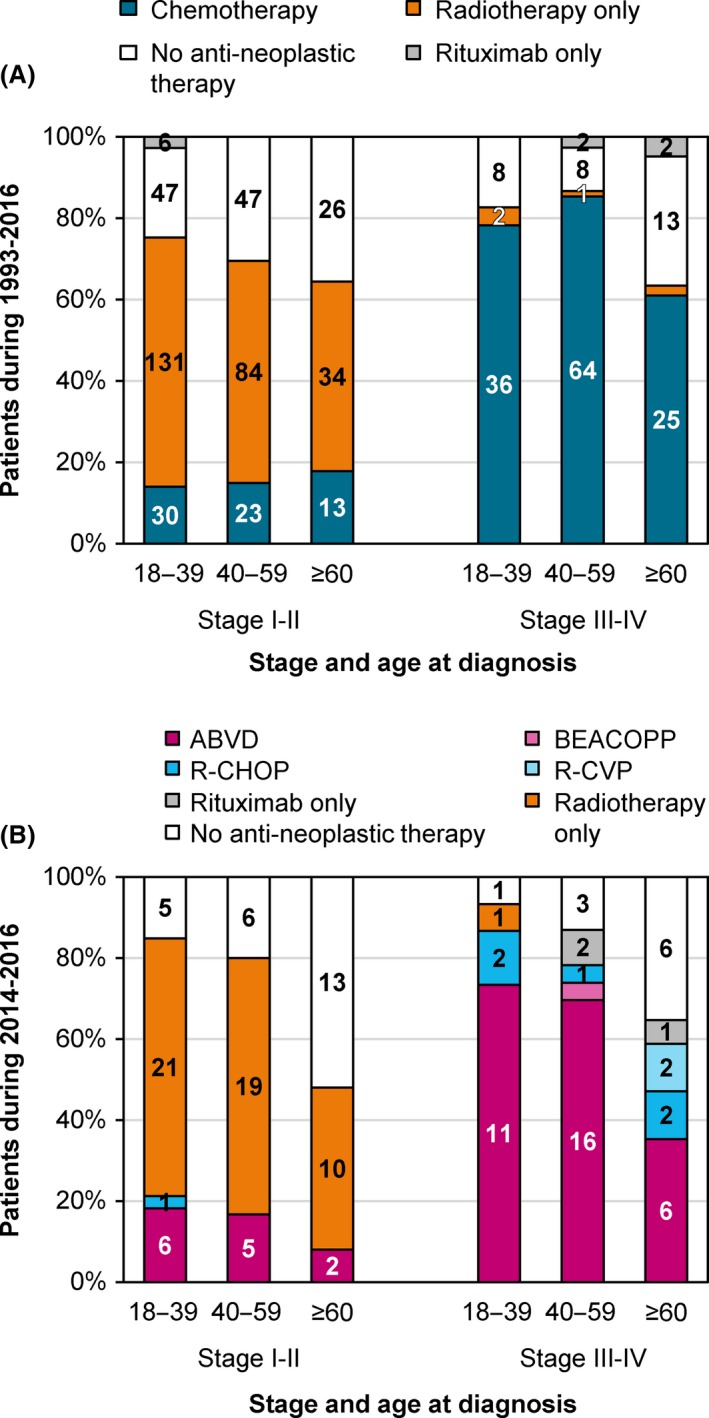
Primary therapy of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to stage and age at diagnosis, 1993–2016. Panel A shows information on primary therapy in broad categories for the entire study period of 1993–2016. Panel B shows more detailed information on primary therapy for the study period of 2014–2016. The absolute number of patients receiving a particular treatment within a specific stage and age group is shown in the graphs. The absolute number and proportion of patients within a specific stage and age group are shown in Tables SII and SIII. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; R‐CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone; R‐CVP, rituximab with cyclophosphamide, vincristine, and prednisone.
At a median follow‐up of 7·7 years (range, 0·1–25·0), 85 (14%) deaths occurred (median age at death, 65 years; range, 32–89 years). RS was generally excellent across the various subgroups studied (Fig 2A–D). A particularly encouraging finding was that patients aged 18–39 years experienced virtually no excess mortality, as compared to the general population (Fig 2C). Also, excess mortality was comparatively low among patients aged 40–59 and ≥60 years (Fig 2C).
Figure 2.
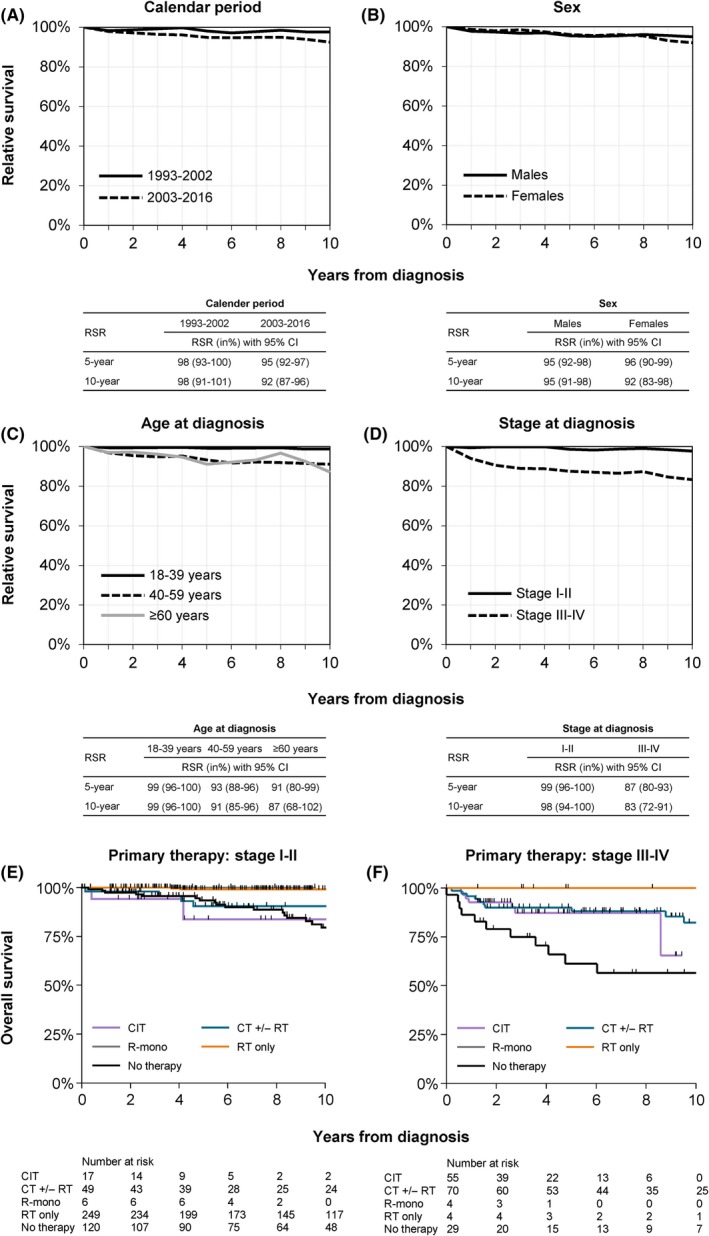
Survival of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands, 1993–2016. Relative survival is shown for the following categories: (A) calendar period of diagnosis, (B) sex, (C) age group, and (D) stage. The tables presented in panels A to D show the projected five‐ and ten‐year relative survival rates (RSRs) with 95% confidence intervals (CIs). Overall survival according to primary therapy among patients with stage‐I/II and ‐III/IV disease is shown in panels E and F, respectively. The projected five‐ and ten‐year overall survival with 95% CIs are presented in Table SV. CIT, chemoimmunotherapy; CT, chemotherapy; RT, radiotherapy; R‐mono, rituximab monotherapy.
The multivariable model, as shown in Table SIV, demonstrated that RS was similar between the two calendar periods (P = 0·891) and sexes (P = 0·477). As expected, the model demonstrated a prognostic effect of age (P = 0·005) and stage (P < 0·001). Interestingly, RS was similar between patients aged 40–59 and ≥ 60 years (P = 0·674).
OS according to the most frequently applied primary therapy merits acknowledgement but not extensive description (Figs 2E,F and Table SV). Ten‐year OS (95% confidence intervals) was 99% (96–100%) for adult patients with limited‐stage disease who received radiotherapy alone and 80% (67–88%) for those with advanced‐stage disease who received treatment with a chemotherapeutic backbone. Furthermore, 10‐year OS (95% confidence intervals) was 80% (69–87%) and 57% (35–74%) respectively among patients with limited‐ and advanced‐stage disease in whom no anti‐neoplastic was applied within the first year after diagnosis. Although limited by small patient numbers, no deaths occurred at a median follow‐up of 5·4 years (range, 1·2–9·6 years) among the ten recipients of rituximab monotherapy (median age, 36 years; age range, 19–72 years).
Discussion
In contrast to a Swedish population‐based study (Molin et al., 2017) — but congruent with a US population‐based study (Shivarov & Ivanova, 2018) — we noted no sex‐based survival differences in NLPHL and no improved survival after the implementation of rituximab into the therapeutic armamentarium of NLPHL. Former studies showed that OS was generally excellent in NLPHL (Molin et al., 2017; Shivarov & Ivanova, 2018; Borchmann et al., 2019). However, for diseases with an indolent course, such as NLPHL, it is appropriate to correct OS for the ES of the general population; that is, RS (Dinmohamed et al., 2018). This is the first study that assessed RS in NLPHL in a large population‐based cohort, showing that the great majority of patients with NLPHL can look forward to a normal life expectancy. Excess mortality in NLPHL might be related to its transformation to a diffuse large B‐cell lymphoma or late sequelae of therapy. To date, strong evidence is lacking whether rituximab could influence the transformation of NLPHL. Therefore, since NLPHL is a rare disease, a large international study with long‐term follow‐up is required to assess the effect of rituximab on the transformation rate.
One of the main strengths of our study includes the use of a nationwide population‐based cancer registry with comprehensive information available for individual patients during a 24‐year period. Furthermore, in contrast to former population‐based studies, we calculated RS as a measure of disease‐specific survival. Limitations mainly pertain to the lack of a central pathology review, detailed information on treatment throughout most of the registry (1993–2013), transformation and relapse rates, and information on cause of death and treatment beyond one year after diagnosis.
In summary, in this large, contemporary, nationwide population‐based study, the incidence of NLPHL increased over time, likely due to enhanced awareness among clinicians and pathologists for the diagnosis of NLPHL and improved diagnostic techniques. Survival among various subgroups of NLPHL patients is largely comparable to the ES of the general population. The implementation of rituximab in current therapeutic regimens does not seem to have augmented survival in our cohort. Future prospective studies in NLPHL are warranted to establish evidence‐based treatment recommendations that consider the delicate balance between efficacy, toxicity, and quality of life.
Authorship contributions
HLAP and AGD designed the study; AGD analysed the data; OV collected the data; HLAP wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Conflict of interest
JMZ and MJK have received travel grants, research support, and honoraria from Roche. The other authors have no conflict of interest to disclose.
Supporting information
Data S1. Supplemental methods.
Fig S1. Age‐specific incidence rates of patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands, 1993–2016. Incidence rates are presented per 1 000 000 person‐years for the following age groups: 0–19, 20–39, 40–59, and ≥60 years. Incidence rates are shown for (A) males and females together, (B) males alone, and (C) females alone.
Fig S2. Age‐specific incidence rates per quinquennial years of age of patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands, 2003–2016. Incidence rates are presented per 1 000 000 person‐years and shown according to sex. The period of 2003–2016 was chosen, as this period in a way represents contemporary clinical practice.
Table SI. Patient characteristics.
Table SII. Primary therapy of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to stage at diagnosis, age at diagnosis, and calendar period of diagnosis, 1993–2016.
Table SIII. Primary therapy of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to stage and age at diagnosis, 2014–2016.
Table SIV. Excess mortality ratio during the first ten years after the diagnosis of nodular lymphocyte‐predominant Hodgkin lymphoma.
Table SV. The projected five‐ and ten‐year overall survival among adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to primary therapy and stage, 1993–2016.
Acknowledgements
The authors thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. Established in 1989, the nationwide population‐based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL).
References
- Alonso, C. , Dutta, S.W. , Mitra, N. , Landsburg, D.J. , Zaorsky, N.G. , Grover, S. , Peterson, J. & Trifiletti, D.M. (2018) Adult nodular lymphocyte‐predominant Hodgkin lymphoma: treatment modality utilization and survival. Cancer Medicine, 7, 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos, I. , Hansmann, M.L. , Franssila, K. , Harris, M. , Harris, N.L. , Jaffe, E.S. , Han, J. , van Krieken, J.M. , Poppema, S. , Marafioti, T. , Franklin, J. , Sextro, M. , Diehl, V. & Stein, H. (2000) European task force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood, 96, 1889–1899. [PubMed] [Google Scholar]
- Borchmann, S. , Joffe, E. , Moskowitz, C.H. , Zelenetz, A.D. , Noy, A. , Portlock, C.S. , Gerecitano, J.F. , Batlevi, C.L. , Caron, P.C. , Drullinsky, P. , Hamilton, A. , Hamlin, P.A. Jr , Horwitz, S.M. , Kumar, A. , Matasar, M.J. , Moskowitz, A.J. , Owens, C.N. , Palomba, M.L. , Younes, A. & Straus, D.J. (2019) Active surveillance for nodular lymphocyte‐predominant Hodgkin lymphoma. Blood, 133, 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.C. , Chin, M.S. , Ng, A.K. , Feng, Y. , Neuberg, D. , Silver, B. , Pinkus, G.S. , Stevenson, M.A. & Mauch, P.M. (2010) Early‐stage, lymphocyte‐predominant Hodgkin's lymphoma: patient outcomes from a large, single‐institution series with long follow‐up. Journal of Clinical Oncology, 28, 136–141. [DOI] [PubMed] [Google Scholar]
- Dickman, P.W. & Adami, H.O. (2006) Interpreting trends in cancer patient survival. Journal of Internal Medicine, 260, 103–117. [DOI] [PubMed] [Google Scholar]
- Dickman, P.W. , Sloggett, A. , Hills, M. & Hakulinen, T. (2004) Regression models for relative survival. Statistics in Medicine, 23, 51–64. [DOI] [PubMed] [Google Scholar]
- Dinmohamed, A.G. , Issa, D.E. , van der Poel, M.W.M. , Schouten, H.C. , Lugtenburg, P.J. , Chamuleau, M.E.D. , Zweegman, S. & Visser, O. (2017) Treatment and relative survival in very elderly patients with DLBCL in The Netherlands: a population‐based study, 1989 to 2015. Blood Advances, 1, 1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed, A.G. , Posthuma, E.F.M. , Visser, O. , Kater, A.P. , Raymakers, R.A.P. & Doorduijn, J.K. (2018) Relative survival reaches a plateau in hairy cell leukemia: a population‐based analysis in The Netherlands. Blood, 131, 1380–1383. [DOI] [PubMed] [Google Scholar]
- Ederer, F. & Heise, H. (1959) Instructions to IBM 650 Programmers in Processing Survival Computations Methodological Note No. 10. End results Evaluation Section. National Cancer Institute, Bethesda, MD. [Google Scholar]
- Eichenauer, D.A. , Fuchs, M. , Pluetschow, A. , Klimm, B. , Halbsguth, T. , Boll, B. , von Tresckow, B. , Nogova, L. , Borchmann, P. & Engert, A. (2011) Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocyte‐predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood, 118, 4363–4365. [DOI] [PubMed] [Google Scholar]
- Eichenauer, D.A. , Aleman, B.M.P. , Andre, M. , Federico, M. , Hutchings, M. , Illidge, T. , Engert, A. & Ladetto, M. (2018) Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 29, iv19–iv29. [DOI] [PubMed] [Google Scholar]
- Molin, D. , Linderoth, J. & Wahlin, B.E. (2017) Nodular lymphocyte predominant Hodgkin lymphoma in Sweden between 2000 and 2014: an analysis of the Swedish Lymphoma Registry. British Journal of Haematology, 177, 449–456. [DOI] [PubMed] [Google Scholar]
- Schouten, L.J. , Hoppener, P. , van den Brandt, P.A. , Knottnerus, J.A. & Jager, J.J. (1993) Completeness of cancer registration in Limburg, The Netherlands. International Journal of Epidemiology, 22, 369–376. [DOI] [PubMed] [Google Scholar]
- Shivarov, V. & Ivanova, M. (2018) Nodular lymphocyte predominant Hodgkin lymphoma in USA between 2000 and 2014: an updated analysis based on the SEER data. British Journal of Haematology, 182, 727–730. [DOI] [PubMed] [Google Scholar]
- Spinner, M.A. , Varma, G. & Advani, R.H. (2019) Modern principles in the management of nodular lymphocyte‐predominant Hodgkin lymphoma. British Journal of Haematology, 184, 17–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Fig S1. Age‐specific incidence rates of patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands, 1993–2016. Incidence rates are presented per 1 000 000 person‐years for the following age groups: 0–19, 20–39, 40–59, and ≥60 years. Incidence rates are shown for (A) males and females together, (B) males alone, and (C) females alone.
Fig S2. Age‐specific incidence rates per quinquennial years of age of patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands, 2003–2016. Incidence rates are presented per 1 000 000 person‐years and shown according to sex. The period of 2003–2016 was chosen, as this period in a way represents contemporary clinical practice.
Table SI. Patient characteristics.
Table SII. Primary therapy of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to stage at diagnosis, age at diagnosis, and calendar period of diagnosis, 1993–2016.
Table SIII. Primary therapy of adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to stage and age at diagnosis, 2014–2016.
Table SIV. Excess mortality ratio during the first ten years after the diagnosis of nodular lymphocyte‐predominant Hodgkin lymphoma.
Table SV. The projected five‐ and ten‐year overall survival among adult patients with nodular lymphocyte‐predominant Hodgkin lymphoma in the Netherlands according to primary therapy and stage, 1993–2016.


