Table 1.
Structure activity relationships of cinchona alkaloid‐derived autophagy inhibitors. IC50 data represents the ability to inhibit autophagy induced by amino acid starvation using EBSS and is mean ± SD of three independent experiments. 2=Autoquin.
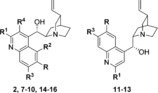
|
Entry |
# |
R |
R1 |
R2 |
R3 |
R4 |
IC50 [μm] |
|---|---|---|---|---|---|---|---|
|
1 |
2 |
OMe |
p‐F‐C6H4 |
H |
H |
H |
0.56±0.15 |
|
2 |
7 a |
OMe |
p‐Cl‐C6H4 |
H |
H |
H |
2.44±1.01 |
|
3 |
7 b |
OMe |
p‐Me‐C6H4 |
H |
H |
H |
2.31±0.07 |
|
4 |
7 c |
OMe |
Ph |
H |
H |
H |
>10 |
|
5 |
7 d |
OMe |
m‐Br‐C6H4 |
H |
H |
H |
7.48±0.70 |
|
6 |
7 e |
OMe |
m‐Cl‐C6H4 |
H |
H |
H |
5.70±0.39 |
|
7 |
7 f |
OMe |
m‐Cl‐p‐F‐C6H3 |
H |
H |
H |
>10 |
|
8 |
9 a |
OMe |
H |
p‐F‐C6H4 |
H |
H |
>10 |
|
9 |
9 b |
OMe |
H |
m‐Cl‐C6H4 |
H |
H |
>10 |
|
10 |
9 c |
OMe |
H |
m‐Cl‐p‐F‐C6H3 |
H |
H |
5.40±2.40 |
|
11 |
9 d |
OMe |
H |
Ph |
H |
H |
>10 |
|
12 |
8 a |
H |
p‐F‐C6H4 |
H |
H |
H |
1.62±0.19 |
|
13 |
8 b |
H |
p‐Cl‐C6H4 |
H |
H |
H |
Toxic |
|
14 |
8 c |
H |
p‐Me‐C6H4 |
H |
H |
H |
>10 |
|
15 |
8 d |
H |
Ph |
H |
H |
H |
>10 |
|
16 |
8 e |
H |
m‐Br‐C6H4 |
H |
H |
H |
>10 |
|
17 |
8 f |
H |
m‐Cl‐C6H4 |
H |
H |
H |
5.43±0.13 |
|
18 |
8 g |
H |
m‐Cl‐p‐F‐C6H4 |
H |
H |
H |
>10 |
|
19 |
10 a |
H |
H |
H |
p‐F‐C6H4 |
H |
>10 |
|
20 |
10 b |
H |
H |
H |
p‐Cl‐C6H4 |
H |
2.52±0.32 |
|
21 |
10 c |
H |
H |
H |
m‐Br‐C6H4 |
H |
>10 |
|
22 |
11 a |
OMe |
p‐F‐C6H4 |
H |
H |
H |
3.30±1.60 |
|
23 |
11 b |
OMe |
p‐Cl‐C6H4 |
H |
H |
H |
2.70±1.30 |
|
24 |
11 c |
OMe |
p‐Me‐C6H4 |
H |
H |
H |
>10 |
|
25 |
11 d |
OMe |
Ph |
H |
H |
H |
>10 |
|
26 |
11 e |
OMe |
m‐Br‐C6H4 |
H |
H |
H |
2.72±1.31 |
|
27 |
11 f |
OMe |
m‐Cl‐C6H4 |
H |
H |
H |
na |
|
28 |
11 g |
OMe |
m‐Cl‐p‐F‐C6H3 |
H |
H |
H |
na |
|
29 |
12 a |
H |
p‐F‐C6H4 |
H |
H |
H |
>10 |
|
30 |
12 b |
H |
p‐Cl‐C6H4 |
H |
H |
H |
5.84±1.30 |
|
31 |
12 c |
H |
p‐Me‐C6H4 |
H |
H |
H |
>10 |
|
32 |
12 d |
H |
Ph |
H |
H |
H |
>10 |
|
33 |
12 e |
H |
m‐Br‐C6H4 |
H |
H |
H |
>10 |
|
34 |
13 a |
H |
H |
H |
p‐F‐C6H4 |
H |
>10 |
|
35 |
13 b |
H |
H |
H |
p‐Cl‐C6H4 |
H |
na |
|
36 |
13 c |
H |
H |
H |
Ph |
H |
>10 |
|
37 |
16 a |
OMe |
H |
H |
H |
p‐F‐C6H4 |
>10 |
|
38 |
16 b |
OMe |
H |
H |
H |
p‐Cl‐C6H4 |
>10 |
|
39 |
16 c |
OMe |
H |
H |
H |
p‐NO2‐C6H4 |
3.87±0.69 |
|
40 |
16 d |
OMe |
H |
H |
H |
p‐CF3‐C6H4 |
3.52±0.69 |
|
41 |
16 e |
OMe |
H |
H |
H |
p‐NHBoc‐C6H4 |
>10 |
|
42 |
16 f |
OMe |
H |
H |
H |
4‐py |
>10 |
|
43 |
16 g |
OMe |
H |
H |
H |
m‐F‐C6H4 |
>10 |
|
44 |
16 h |
OMe |
H |
H |
H |
m‐F‐p‐F‐C6H4 |
>10 |
|
45 |
16 i |
OMe |
H |
H |
H |
3,5‐di‐F‐ C6H4 |
>10 |
|
46 |
16 j |
OMe |
H |
H |
H |
m‐Cl‐p‐F‐C6H4 |
>10 |
|
47 |
16 k |
OMe |
H |
H |
H |
3,4‐di‐OMe‐ C6H4 |
>10 |
|
48 |
16 l |
OMe |
H |
H |
H |
3,5‐di‐OMe‐ C6H4 |
>10 |
|
49 |
14 |
OMe |
H |
H |
H |
‐Br |
>10 |
