Figure 1.
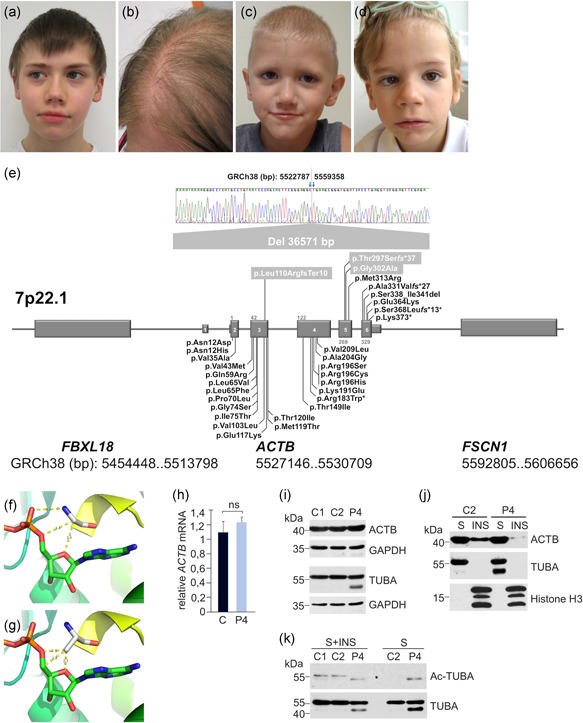
Clinical findings, ACTB variant compilation, and effects of the ACTB p.(Gly302Ala) variant. (a) Patient 1 at the age of 11 years. (b) Patient 2 at the age of 35 years. (c) Patient 3 at the age of 6 years. (d) Patient 4 at the age of 4 years. Shared facial dysmorphism consists of wavy interrupted eyebrows, dense eyelashes, wide nose, wide mouth, prominent cheeks, and chin. Sparse scalp hair in patients 2 and 4. (e) Compilation of novel and reported ACTB variants associated with BWCFF, putative ACTB loss‐of‐function and ACTB‐AST (chromosomal order of genes and exons not drawn to scale). Modeling of ACTB residue Gly‐302 (f) and variant Ala‐302 (g). (h) The relative ACTB messenger RNA expression in a healthy donor (C) and Patient 4 (P4) was assessed by quantitative real‐time polymerase chain reaction using the method. Values were normalized to the amount of human ribosomal protein L32 mRNA. Immunoblot analysis of ACTB and α‐tubulin (TUBA) in total lysates, NP‐40 soluble (S) and insoluble (INS) fractions of blood mononuclear cells from healthy individuals (C1 and C2) and Patient 4 (P4). GAPDH and histone H3 were used as loading controls (i,j). (k) TUBA acetylation was analyzed in total lysates (S + INS) and NP‐40 soluble (S) fraction obtained from control and Patient 4 blood mononuclear cells using anti‐Ac‐TUBA and anti‐TUBA antibodies. BWCFF, Baraitser‐Winter cerebrofrontofacial syndrome
