Scheme 1.
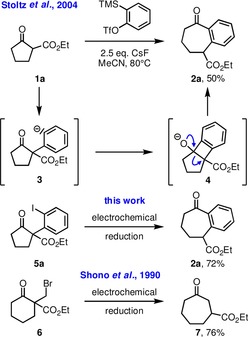
Previously reported ring‐transformation of oxoester 1 a with an in situ generated aryne4 via intermediates 3 and 4. Entry into the same reaction sequence leading to product 2 a by electrochemical reduction of iodoarene 5 a (this work) and electrochemical ring‐enlargement of oxoester 6 by cathodic reduction. Conditions: 3 equiv TMSCl, nEt4NOTos, DMF, 23 °C, 0.2 A, 4 F mol−1, Pb cathode, carbon anode.8
