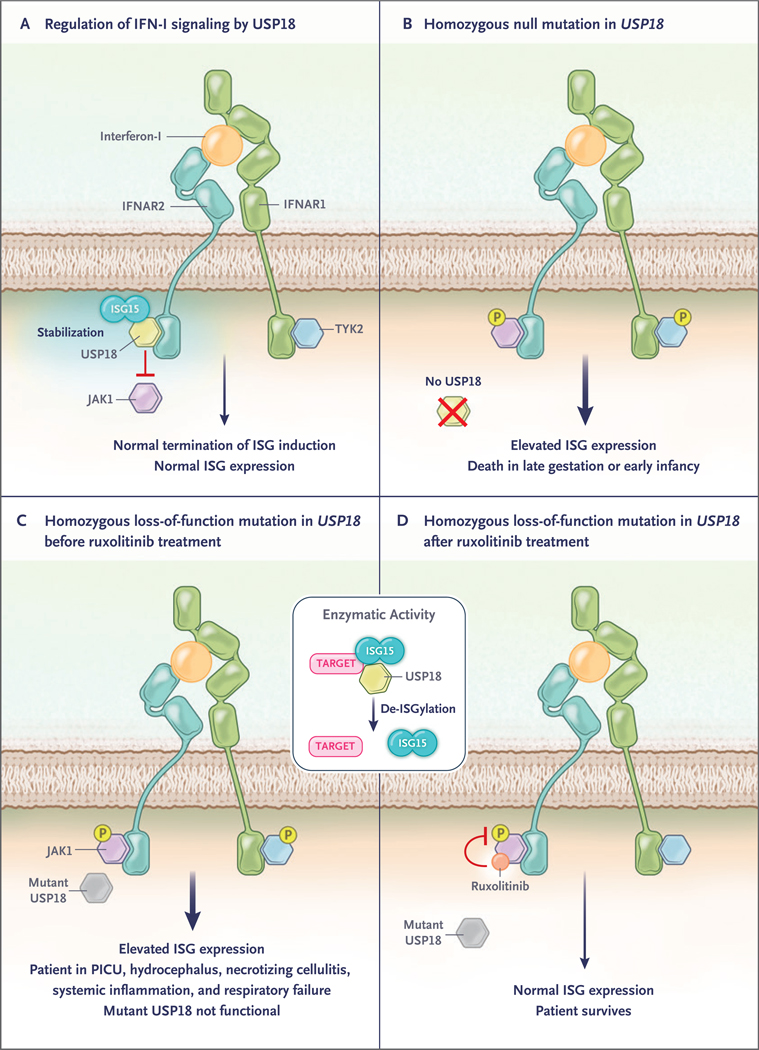
Figure 1 (facing page). Molecular Functions of USP18.
Type I interferon (IFN–I) binds IFN–I receptor, consisting of two subunits, IFN–I receptor 1 (IFNAR1) and IFN–I receptor 2 (IFNAR2). It signals through activation of two receptor–associated protein tyrosine kinases, Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2), which phosphorylate (P) signal transducer and activator of transcription 1 (STAT1) and 2 (STAT2) to induce the transcription of hundreds of interferon–stimulated genes (ISGs). Panel A shows the activity of USP18 (an ISG) as a negative–feedback regulator of IFN–I signaling through the inhibition of JAK1 recruitment to IFNAR2. ISG15 stabilizes USP18 and protects it against proteasomal degradation, resulting in normal ISG expression. Panel B shows that homozygous mutations resulting in no synthesis of USP18 cause unrestrained IFN–I signaling and are lethal. Panels C and D show the outcome in the patient in the case report before and after the initiation of ruxolitinib, a JAK1 inhibitor. The box in the middle of the figure shows that in addition to inhibiting JAK1, USP18 has catalytic activity: it cleaves the ubiquitin–like ISG15 protein from conjugated proteins in a process called de–ISGylation. PICU denotes pediatric intensive care unit.