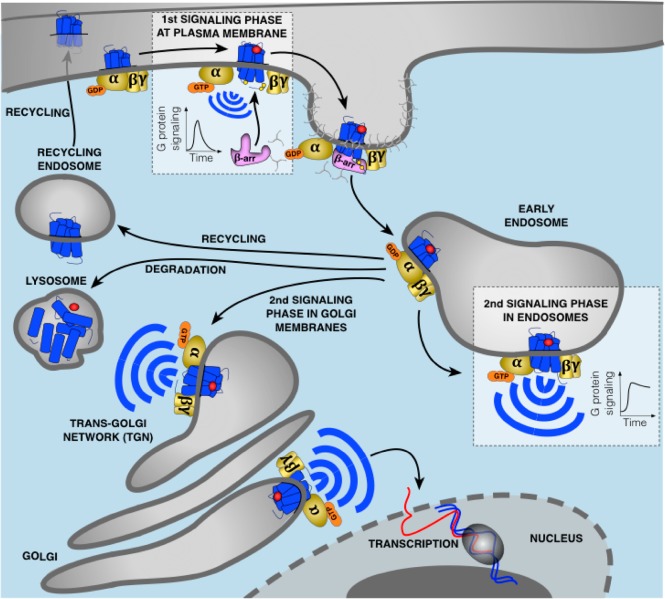
Figure 1.
Updated model of G protein signaling. Agonist binding to GPCRs is detected by the Gα subunit of heterotrimeric G proteins. This induces exchange of GDP for GTP causing dissociation of the GTP-bound Gα from Gβγ which both activate membrane-localized effectors. This signaling is followed by the phosphorylation of the receptor by GRKs leading to recruitment of β-arr to the phosphorylated receptor. This event causes G protein uncoupling from the receptor and signaling desensitization. Recruitment of β-arr also promotes GPCR internalization into CCPs and receptor trafficking to early endosomes where desensitized receptors dissociate from β-arr and recycle back to the plasma membrane, are directed to lysosomes for degradation, or can undergo another round of G protein activation from early endosomes or Golgi membranes. In contrast to G protein signaling at the plasma membrane which is rapidly dampened by β-arr, this second activation upon GPCR internalization is generally more sustained. The particular duration and location of G protein signaling is critical for many cellular processes.