Figure 2. Structure of the caspase-3 heterotetramer.
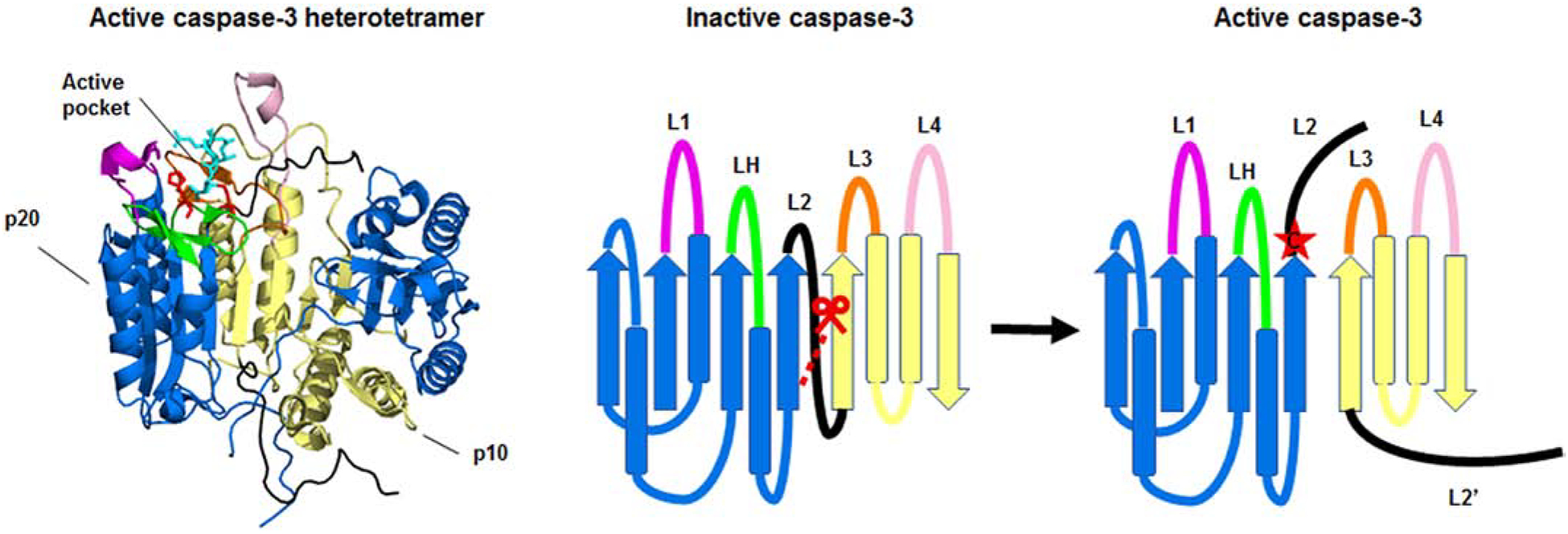
The crystal structure of the active caspase-2 heterodimer (left) and a ribbon diagram of the structure (right) are shown. The large (p20) and small (p10) subunits of the heterodimer are depicted in blue and yellow respectively. Caspase-3 is shown bound to the inhibitor z-DEVD-cmk (cyan). The catalytic histidine and cysteine are shown in red. The loops, L1 (magenta), L2 (black), LH (green), L3 (orange), and L4 (pale pink) make up the active pocket. Caspase-3 is activated by cleavage of the intersubunit linker (L2). Cleavage of the intersubunit linker opens up the active pocket, exposing the active cysteine (red star). For clarity, the loops, catalytic residues, and inhibitor are only highlighted in one half of the heterotetramer. The molecular models of the protein structures were created using PyMOL v1.3.Edu with the full length caspase-3 crystal structure (PDB:2DKO) [38].
