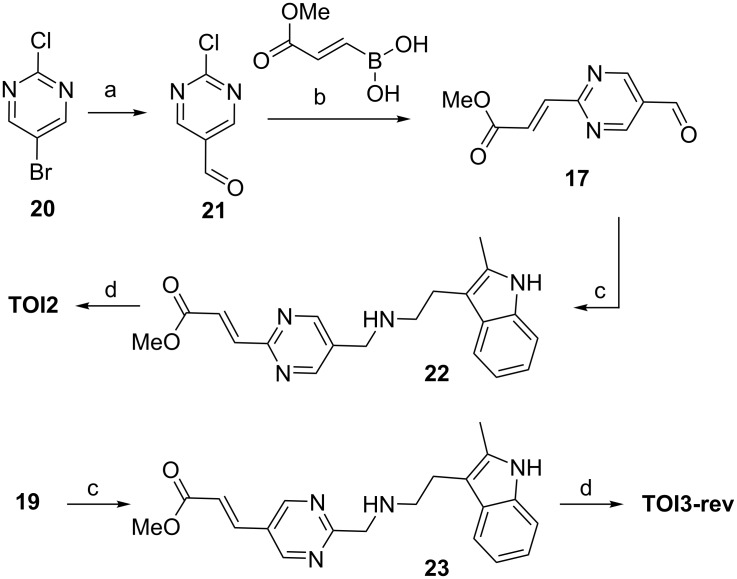
Scheme 3.
Reaction conditions: a) 5-bromo-2-chloropyrimidine (1 equiv), ethyl formate (1.5 equiv), THF (20 mL), n-butyllithium (0.6 equiv, 2.5 M) in hexane, −100 °C, 2 h, 42%; b) boronic acid (1.3 equiv), PdCl2(PPh3)2 (0.1 equiv), dioxane/water (8:2), Na2HPO4 (2.0 equiv), TEA (4.0 equiv), 95 °C, 15 h, 40%; c) indolamine 10 (1.1 equiv) DCE, STAB (1.0 equiv), TEA (2 equiv), rt, 61% for 22, 68% for 23; d) NaOH at −10 °C, NH2OH·H2O at −10 °C, MeOH, rt, 12 h, 49% for TIO2 and 51% for TIO3-rev.