Table 1.
Representative screening results of the asymmetric aziridination reaction of PhSO2CF2CHN2.a
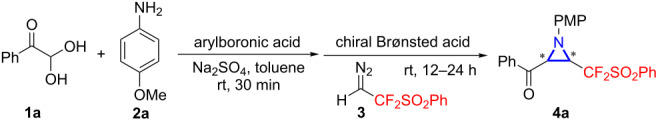 | ||||
| entry | arylboronic acid (mol %) | chiral Brønsted acid (mol %) | yield of 4a (%)b | ee (%) of 4a and dr of crude mixturec |
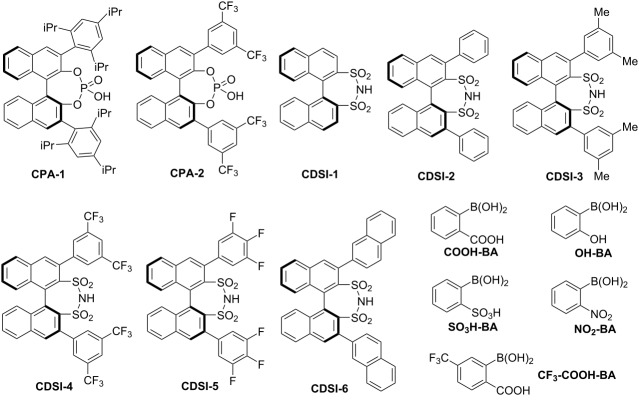
| 1 | COOH-BA (8) | CPA-1 (5) | 24 | 51, 13:1 |
| 2 | COOH-BA (8) | CPA-2 (5) | 28 | 25, 11:1 |
| 3 | COOH-BA (8) | CDSI-1 (5) | 21 | 41, 19:1 |
| 4 | COOH-BA (8) | CDSI-2 (5) | 50 | 41, 9:1 |
| 5d | COOH-BA (8) | CDSI-3 (5) | 16 | 60, 5:1 |
| 6d | COOH-BA (8) | CDSI-4 (5) | 64 | 73, 13:1 |
| 7 | COOH-BA (8) | CDSI-5 (5) | 34 | 33, 10:1 |
| 8 | COOH-BA (8) | CDSI-6 (5) | 47 | 52, 9:1 |
| 9 | OH-BA (8) | CDSI-4 (5) | 63 | 68, 28:1 |
| 10d | SO3H-BA (8) | CDSI-4 (5) | 62 | 66, 16:1 |
| 11d | NO2-BA (8) | CDSI-4 (5) | 45 | 62, 16:1 |
| 12 | CF3-COOH-BA (8) | CDSI-4 (5) | 81 | 67, 8:1 |
| 13e | COOH-BA (8) | CDSI-4 (5) | 60 | 47, 5:1 |
| 14f | COOH-BA (8) | CDSI-4 (5) | trace | n.d. |
| 15d | COOH-BA (8) | CDSI-4 (10) | 65 | 70, 12:1 |
| 16d | – | CDSI-4 (5) | 10 | 60, >20:1 |
aGeneral reaction conditions: 1a (8 mg, 0.05 mmol, 1.0 equiv), 2a (7 mg, 0.055 mmol), arylboronic acid (0.004 mmol), and Na2SO4 (40 mg) was stirred in toluene (1 mL) at rt for 30 min, then the chiral Brønsted acid (0.0025 mmol) and 3 (18 mg, 0.075 mmol) were added and the mixture was reacted at rt for 12 hours unless otherwise noted; byield of isolated product 4a was given for entries labelled with d; hexafluorobenzene was used as an internal standard to determine the yield in other cases; cee of 4a was determined by chiral HPLC analysis, and the dr of the crude reaction mixture was probed by 19F NMR analysis; d0.3 mmol scale of reaction was conducted: 1a (46 mg, 0.3 mmol, 1.0 equiv), 2a (41 mg, 0.33 mmol), arylboronic acid (0.024 mmol), and Na2SO4 (200 mg) was stirred in toluene (2 mL) at rt for 30 min, then the chiral Brønsted acid (0.015 mmol) and 3 (105 mg, 0.45 mmol) were added and the mixture was reacted at rt for 12–24 hours; eCH2Cl2 was used as the solvent; freaction was operated at 0 °C.
