Fig. 3. Regulatory roles of the M. tuberculosis proteasome.
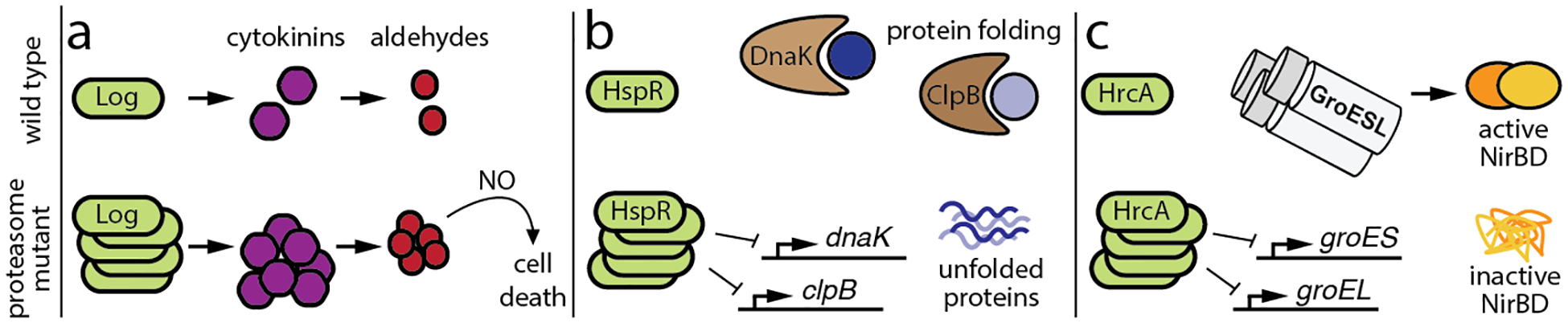
(a) Log is a pupylated proteasome substrate that synthesizes cytokinins, which break down into aldehydes. In the absence of proteasomal degradation, accumulation of Log leads to the overproduction of cytokinins, and the ensuing accumulation of aldehydes synergizes with nitric oxide (NO) to kill M. tuberculosis (Samanovic et al. 2015). (b) HspR, a repressor of M. tuberculosis genes encoding the protein-folding chaperones DnaK and ClpB, is normally degraded by the PafE-proteasome. In the absence of PafE, accumulation of HspR leads to the repression of chaperone genes, which is associated with defective growth and an inability to fully withstand heat shock (Jastrab et al. 2017; Jastrab et al. 2015). (c) M. tuberculosis HrcA represses the genes encoding the GroES/GroEL chaperonins, which are required for the full activity of the nitrite reductase NirBD. It is proposed that failure to pupylate and degrade HrcA prevents full chaperonin expression, leading to a loss of NirBD activity; this prohibits M. tuberculosis from using nitrate as a nitrogen source (Becker et al. 2019).
