Table 1. |.
Exploration of reaction conditions that enables remote site-selective arylation of benzoazins.
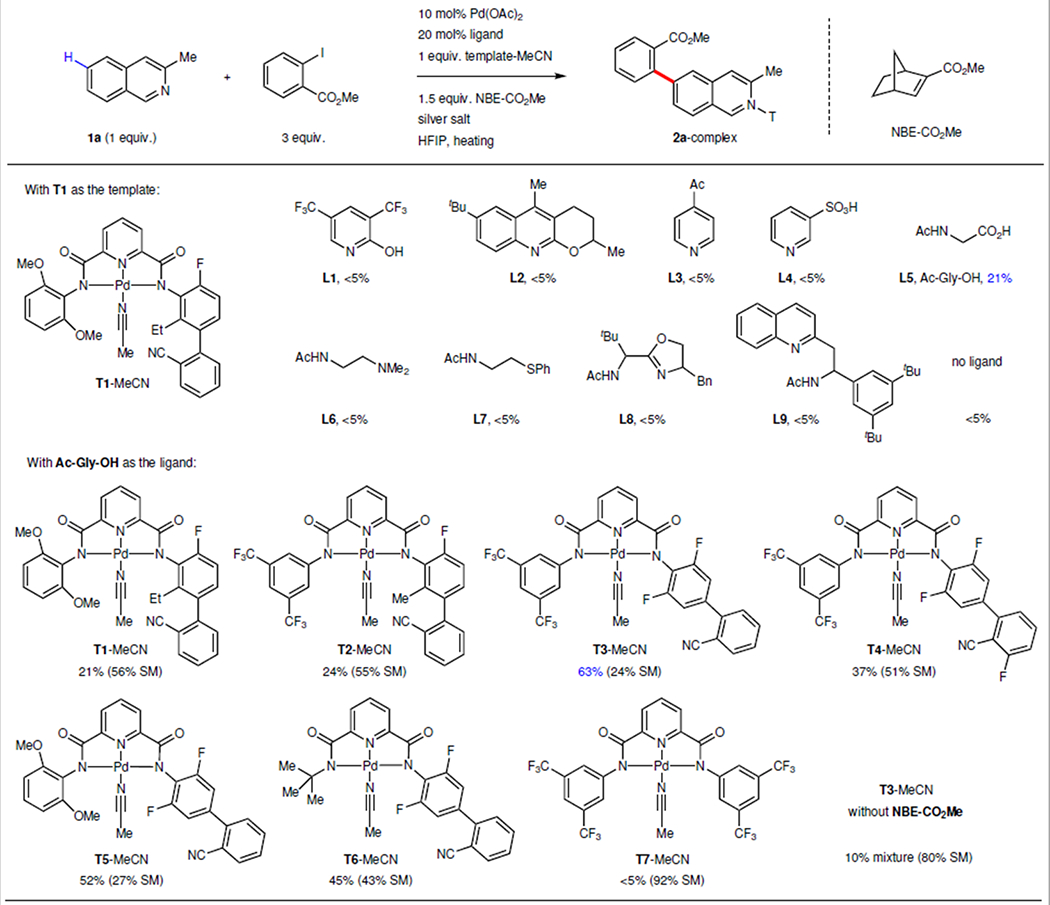 |
10 mol% Pd(OAc)2, 20 mol% ligand, 1 equiv. 3-methyl isoquinoline, 1 equiv. template–MeCN, 1.5 equiv. NBE–CO2Me, 3 equiv. methyl 2-iodobenzoate, 3 equiv. AgOAc, 1 equiv. Ag2CO3, HFIP, 80 °C. The yield was determined by 1H NMR spectroscopy. T, template; HFIP, hexafluoro-2-propanol; Me, methyl group; Et, ethyl group; Ph, phenyl group; Ac, acetyl group; CN, cyano group.
