Table 3. |.
Remote C7-arylation of tetrahydroisoquinolines.
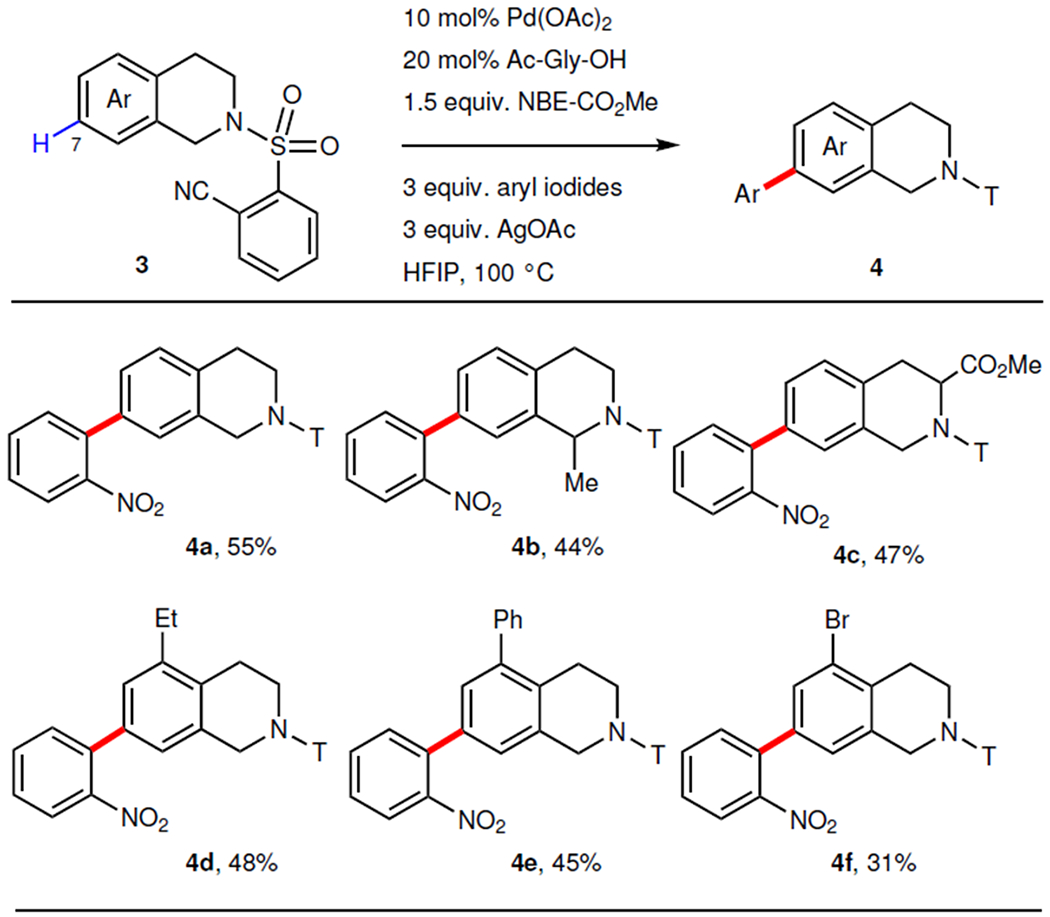 |
Aryl iodide = 1-iodo-2-nitrobenzene. 10 mol% Pd(OAc)2, 20 mol% Ac–Gly–OH, 1 equiv. tetrahydroisoquinolines, 1.5 equiv. NBE–CO2Me, 3 equiv. aryl iodide, 3 equiv. AgOAc, HFIP, 100 °C. For 4c and 4e, 20 mol% Pd(OAc)2 and 40 mol% Ac–Gly–OH were used. For each entry number (in bold), data were reported as isolated yield.
