Abstract
This paper reports the synthesis, characterization, anticancer screening and quantum chemical calculation of a tetradentate Schiff base 2,2'-((1E,1'E)-((2,2-dimethylpropane-1,3-diyl)bis- (azanylylidene))bis(methanylylidene))bis(4-fluorophenol) (L2F) and its Pd (II) complex (PdL2F). The compounds were characterized via UV-Visible, NMR, IR spectroscopy and single crystal x-ray diffraction. Density Functional Theory (DFT) and time-dependent DFT calculations in gas and solvent phases were carried out using B3LYP, B3P86, CAM-B3LYP and PBE0 hybrid functionals combined with LanL2DZ basis set. Complexation of L2F to form PdL2F was observed to cause a bathochromic shift of the maximum absorption bands of n–π* from 327 to 410 nm; an upfield shift for δ (HC = N) from 8.30 to 7.96 ppm and a decreased wavenumber for ν(C = N) from 1637 to 1616 cm-1. Overall, the UV-Vis, NMR and IR spectral data are relatively well reproduced through DFT and TD-DFT methods. L2F and PdL2F showed IC50 of 90.00 and 4.10 μg/mL, respectively, against human colorectal carcinoma (HCT116) cell lines, signifying increased anticancer activity upon complexation with Pd (II).
Introduction
Schiff bases, also known as imines (C = N) or azomethines (HC = N) were first reported by Hugo (Ugo) Schiff (1834–1915) in 1864 [1]. They are commonly formed through the condensation reaction of primary amines with aldehydes or less commonly, ketones [2]. The resultant imines (R1HC = N–R2 or R1R2C = N–R3), where R1, R2 and R3 can be any aryl or alkyl groups, can form complexes with metal ions through donation of the lone pair of electrons of nitrogen. One of the most regularly reported NNOO ligands are the salen-type ligands, formerly termed for a family of bisimine compounds, N, N′-bis (salicylidine) ethylenediamine derived from salicylaldehyde and ethylenediamine in 2:1 molar ratio [3]. In evolution, the family of the salen-type compounds is not only limited to ligands derived from ethylenediamine but also used to describe ligands derived from other primary diamines such as propanediamine and phenylenediamine. The products of the former are also known as salpn-type ligands and the latter are denoted as salophen-type ligands. This paper focuses on the former type of salen and its corresponding palladium (II) complex.
Schiff base ligands have become progressively popular in coordination chemistry owing to their capability to coordinate with many transition metals, stabilizing them in multiple oxidation numbers. This exceptional chelating ability is mainly empowered by the presence of azomethine nitrogen that carries a lone pair of electrons situated in an sp2 hybrid orbital [4]. The effectiveness of Schiff bases as chelating agents is enhanced with the presence of O-H or S-H functional group(s) within 2–3 atomic distances from the azomethine group [5,6]. The stability of Schiff base complexes is largely enhanced through the chelate effect of the polydentate ligands.
Schiff bases are known for their wide potential as bioactive agents such as anticancer [7], antifungal [8,9] and antileishmanial [10]. Previously, we reported the structure-antioxidant activity relationship of a series of phenolic Schiff bases as free radical scavengers using both experimental and DFT calculations [11]. The complexation of Schiff bases as privileged ligands with metals increases their applicability in both chemical and biological processes [12]. Recently, we investigated the bioactivity of an N,O bidentate Schiff base, (E)-(4-methoxybenzylimino)methyl)phenol and its Ni(II) and Pd(II) complexes against HCT116 colorectal cancer cells and Escherichia coli, and the obtained results displayed that the parent ligand is a more superior anticancer and antibacterial agent than positive control [13].
Quantum chemical calculations are considered powerful tools to verify spectral data including the prediction of 1H and 13C NMR chemical shifts [14–16], UV-Visible absorption bands [17–19] and X-ray structure parameters [20, 21]. Reported studies proved that to predict the excited states of some natural compounds, the use of B3LYP and PBE0 hybrid functionals are appropriate to estimate the excited state energies [22–26]. Previously, we used B3P86 and B3LYP hybrid functionals to predict the maximum absorption bands of a series of natural polyphenols [27]. Lumpi et al. (2013) showed that the M06-2X hybrid functional was suitable to predict the absorption and emission spectra of oligothiophene-based compounds [28]. Quartarolo and Russo applied PBE0 and ab initio multi-reference coupled cluster with the resolution of identity approximation (RICC2) approaches to predict the UV-Visible spectra of pyranoanthocyanins, a class of derived anthocyanin molecules; they showed that the use of larger basis sets results in little improvement of excitation energies, and that the conformational effect has a slight influence on the λMAX predictions [29]. In another study, Sousa et al. (2012) tested B3LYP and PBE0, and long-range corrected ωB97X and ωB97XD hybrid functional to predict the absorption electronic spectra of the isopentaphyrin derivative and its lutetium complex; and they showed that ωB97XD is the most reliable to reproduce the absorption electronic spectra of the isopentaphyrin derivative and its lutetium complex [30]. In regard to the 1H and 13C chemical shift calculations, the gauge-independent atomic orbital (GIAO) method is one of the most common approaches used to predict nuclear magnetic shielding tensors (σiso) [31, 32].
The present study aims at investigating anticancer activity of a synthesized Schiff base L2F and its palladium (II) complex PdL2F. The ligand and its complex were characterized by single crystal x-ray diffraction, NMR, IR and UV-Vis spectroscopic techniques. To support the experimental data, DFT and TD-DFT calculations in gas and solvent phases were carried out to predict maximum absorption bands, vibrations modes and chemical shifts of L2F and PdL2F. In addition, the chelation effect of L2F on the experimental data is emphasized.
Material and methods
Starting materials and instruments
All chemicals and solvents purchased from commercial suppliers were used without further purification. The elemental analysis (C, H, and N) of ligand, L2F and complex, PdL2F were obtained from Thermo Scientific Flash 2000 Elemental Analyser. Melting points were determined using Stuart SMP10. Perkin-Elmer Spectrum One FTIR spectrometer using KBr pellets recorded infrared spectra of ligand, L2F and complex, PdL2F between 450–4000 cm-1. 1H and 13C NMR spectra were recorded on a Bruker Varian 600 MHz spectrometer as CDCl3 or DMSO-d6 solutions.
X-ray single crystal data for the coordination polymers were collected at 293(2) and 171(1) K on Bruker D8 QUEST with photon CCD area-detector diffractometer Mo-Kα (λ = 0.71073 Å). High-quality crystals were chosen using a polarizing microscope and mounted on a glass fibre. Data processing and absorption correction was performed using a multi-scan method. The structures were solved by direct method using SHELXS. All data were refined by full matrix least-squares refinement against |F2| using SHELXL [33], and the final refinement include atomic position for all the atoms, anisotropic thermal parameters for all the non-hydrogen atoms, and isotropic thermal parameters for the hydrogen atoms. The programs PLATON and Mercury were used throughout the study [34].
Synthesis
Synthesis of Schiff base L2F
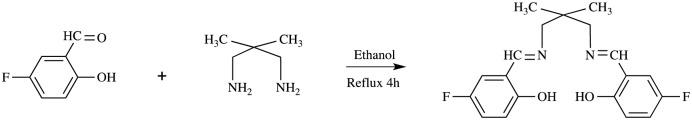
L2F was prepared by adding a 10-mL hot ethanolic solution of DMPD (1 mmol, 0.1022 g) into a stirred equivolume ethanolic solution of 5-fluorosalicylaldehyde (2 mmol, 0.2802 g) (Fig 1). The solution was refluxed over a period of 4 h, cooled to room temperature and chilled overnight. The yellow precipitate obtained was filtered off, washed with cold EtOH and air-dried. The yellow single crystal of the compound was obtained upon slow evaporation from ethanol. The synthesized compound was identified as 2,2'-((1E,1'E)-((2,2-dimethylpropane-1,3-diyl)bis(azanylylidene))bis(methanylylidene))bis(4-fluorophenol), a tetradentate Schiff base assigned as L2F. Yellow solid; yield, 85.0%; m.p. 115–116 °C. Elemental analysis for L2F, analysed as C19H20F2N2O2 (326.28 gmol-1); % Found (Calc.) C, 65.81 (65.88): H, 5.80 (5.82); N, 8.23 (8.09); UV-Vis bands (MeCN, nm): π-π* (C = N) 257, n-π* (C = N) 327; IR bands (KBr pellet, cm-1): ν(O-H) 3271, ν(C = N) 1637, ν(C-O) 1071, ν(C = C) 1583, ν(C-H sp2) 3071, ν(C-H sp3) 2956; 1H NMR (500 MHz, CDCl3, ppm): δ(OH) 13.25 (s, 1H), δ(HC = N) 8.30 (s, 1H); 13C NMR (500 MHz, CDCl3, ppm): δ(C-OH) 157.74, δ(C = N) 164.74.
Fig 1. Synthesis of L2F.
Synthesis of complex PdL2F
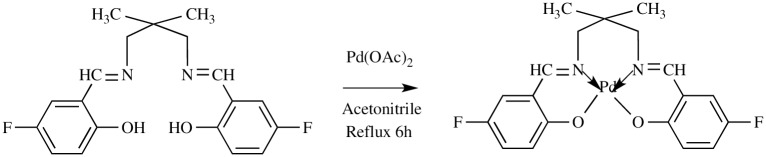
PdL2F was synthesized by dissolving palladium (II) acetate (1 mmol, 0.2248 g) in 20 mL of acetonitrile in a round bottom flask.1mmol, 0.3464 g of L2F was dissolved separately in 20 mL of acetonitrile (Fig 2). The ligand solution was added dropwise into the flask containing the metal salt solution with stirring, then refluxed for 6 h. The dark yellow precipitate was filtered off, washed with a small amount of cold acetonitrile and air-dried. The yellow single crystal of the complex was obtained upon slow evaporation of DMSO: MeOH (1:1 v/v). The synthesized compound was identified as 2,2'-((1E,1'E)-((2,2-dimethylpropane-1,3-diyl)bis-(azanylylidene))bis(methanylylidene))bis(4-fluorophenol)palladium(II), a tetradentate Schiff base complex denoted as PdL2F. Yellow solid; yield, 55.5%; m.p. 295–300 °C. Elemental analysis for PdL2F, analysed as C19H18F2N2O2Pd (450.78 gmol-1); % Found (Calc.) C, 50.10 (50.63): H, 3.92 (4.03); N, 6.92 (6.21); UV-Vis bands (MeCN, nm): π-π* (C = N) 255, n-π* (C = N) 410; IR bands (KBr pellet, cm-1): ν(C = N) 1616, ν(C-O) 1079, ν(C = C) 1545, ν(C-H sp2) 3041, ν(C-H sp3) 2960; 1H NMR (500 MHz, CDCl3, ppm): δ(HC = N) 7.96 (s, 1H); 13C NMR (500 MHz, CDCl3, ppm): δ(C = N) 164.40.
Fig 2. Synthesis of PdL2F.
Cytotoxicity test
The HCT 116 (ATCC, CCL247) colorectal carcinoma cells were obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA. The cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% heat activated fetal bovine serum and 1% penicillin-streptomycin at 37°C in a 5% CO2 atmosphere. The HCT116 cells were seeded at 2,000 cells/ 200 μL onto each well of the 96-well plate and were treated with Schiff base ligand (L2F) and palladium(II) (PdL2F) (0.01–100 μg/mL) followed by incubation for 72 hours at 37°C and 5% CO2 overnight. DMSO (0.001–10%) and 5-FU (0.01–100 μg/mL) were also included as vehicle and positive controls, respectively. Treated cells were fixed with 10% (w/v) tricholoracetic acid (TCA) at 4 °C for 30 minutes. The fixed cells were washed with tap water. Each well was stained with 0.4% (w/v) SRB solution at room temperature for 10–15 minutes. The plate was rinsed with (1% v/v) acetic acid for the removal of the unbound dye and left to dry overnight. Tris base (10 mM) was added for dye solubilisation. The plate was read at 570 nm. Data obtained was used to plot the dose-response curve from which IC50 was determined. IC50 value is defined as the concentration of a test compound required to achieve half maximal inhibition [35].
Theoretical details
Geometry optimisation and frequency calculations of L2F Schiff base and its corresponding complex with palladium metal were performed using DFT method. Five hybrid functionals B3LYP, B3P86, CAM-B3LYP and PBE0 combined with LanL2DZ basis set were tested [36]. The true minima of the optimized structure were confirmed by the absence of imaginary frequencies. The calculated vibrational modes were scaled by a factor of 0.9679 [37]. Excited states (ES) calculations were performed using TD-DFT method. The maximum absorption bands, vertical electronic excitations and oscillator strengths (f>0 for allowed transition) were calculated using the five hybrid functions [38, 39]. The predicted magnetic isotropic shielding tensors (σ) were calculated using the standard Gauge-Independent Atomic Orbital approach (GIAO) [40], using the hybrid functional B3LYP combined with LanL2DZ basis set. The isotropic shielding values were used to calculate the isotropic chemical shifts δ with respect to the reference tetramethylsilane (Si(CH3)4). δiso(X) = σTMS(X)–σiso(X), where δiso is isotropic chemical shift and σiso isotropic shielding constant. The predicted chemical shifts were obtained using the equation δexp = aδcal+b, where δcal = δiso. The solvation effect was considered implicitly using polarisable continuum model (PCM) [41]. In such model, the tilted substrates were embedded into a shape-adapted cavity surrounded by a dielectric continuum solvent, described by its dielectric constant (e.g., εCDCl3 = 4.7113). The PCM has been reported to correctly model major solvent effects such as electrostatic effects of the medium, providing no specific solute-solvent interactions such as hydrogen bond interactions, dipole-dipole interactions, or induced dipole-dipole interactions [42]. For excited state calculations, the solvent effects were considered by using IEF-PCM and state-specific solvation (SS-PCM) [43, 44]. DFT calculations were performed using Gaussian09 package [45].
Results and discussion
UV-visible spectroscopy
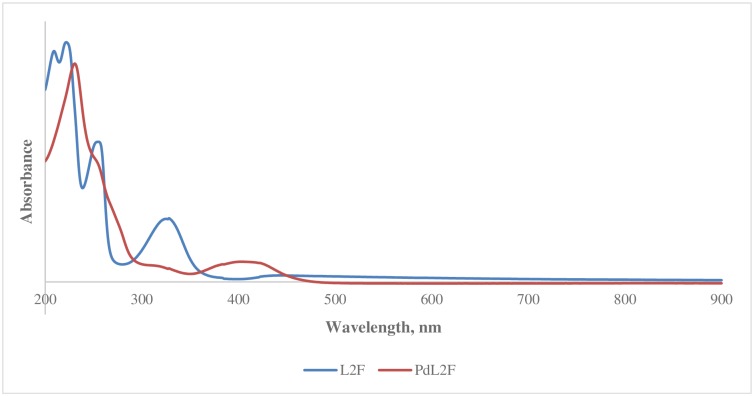
The main experimental and predicted maximum absorption bands of L2F Schiff base and PdL2F are shown in Tables 1 and 2. The observed λMAX at 257 nm as shown in Fig 3 is attributed to π-π* electronic transition of the C = N chromophore, and it is in accordance with the value reported by Khanmohammadi, Salehifard, & Abnosi, (2009) [46]. Theoretically, this band corresponds to an electronic transition between HOMO and LUMO orbitals. As can be seen from Table 3, this band was well reproduced with the B3LYP and B3P86 hybrid functionals in gas, IEF-PCM and SS-PCM phases (Table 1). For instance, by using B3LYP hybrid functional for L2F, variations of 2, 0 and 3 nm with respect to the experimental value were obtained in gas, IEF-PCM and SS-PCM phases, respectively. This band was slightly influenced by the solvatochroism effect where both models IEF-PCM and SS-PCM showed negligible shifts of the corresponding value obtained in gas phase with both B3LYP and B3P86 hybrid functional with variation less than 3 nm. The solvent effect may arise from intermolecular H-bonding or weaker van der Waal’s forces between the F and O-containing Schiff base with the solvent, acetonitrile. CAM-B3LYP and PBE0 failed in the reproduction of the experimental value of the observed band at 275 nm. CAM-B3LYP and PBE0 hybrid functionals for L2F underestimated the experimental value with variations of 22 and 10 nm in gas phase, respectively. The complexation of the Schiff base ligand L2F with Pd (II) has an effect on the observed band at 257 nm with slight hypsochromic shift (blue shift) of 2 nm. Similarly, the band at 255 nm of PdL2F complex was well reproduced with B3P86 and B3LYP, while it is underestimated with CAM-B3LYP and PBE0 hybrid functionals with variations of 32 and 8 nm, respectively.
Table 1. λMAX (nm), EMAX (eV), f of π→π* transitions of L2F and PdL2F calculated using B3LYP, PBE0, CAM-B3LYP and PBE0 hybrid functional in gas, IEF-PCM and SS-PCM.
| Gas | IEF-PCM | SS-PCM | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λMAX | EMAX | F | λMAX | EMAX | f | λMAX | EMAX | f | λMAX | EMAX | |
| B3LYP | |||||||||||
| L2F | 259 | 4.79 | 0.29 | 257 | 4.82 | 0.48 | 260 | 4.77 | 0.67 | 257 | 4.82 |
| PdL2F | 259 | 4.78 | 0.20 | 258 | 4.80 | 0.39 | 261 | 4.75 | 0.60 | 255 | 4.86 |
| B3P86 | |||||||||||
| L2F | 258 | 4.81 | 0.28 | 256 | 4.84 | 0.47 | 259 | 4.79 | 0.67 | 257 | 4.82 |
| PdL2F | 256 | 4.84 | 0.16 | 254 | 4.87 | 0.32 | 257 | 4.83 | 0.48 | 255 | 4.86 |
| CAM-B3LYP | |||||||||||
| L2F | 235 | 5.28 | 0.38 | 234 | 5.29 | 0.46 | 237 | 5.23 | 0.62 | 257 | 4.82 |
| PdL2F | 223 | 5.57 | 0.68 | 232 | 5.35 | 0.65 | 236 | 5.25 | 0.93 | 255 | 4.86 |
| PBE0 | |||||||||||
| L2F | 247 | 5.01 | 0.47 | 247 | 5.02 | 0.58 | 250 | 4.95 | 0.77 | 257 | 4.82 |
| PdL2F | 247 | 5.02 | 0.25 | 245 | 5.05 | 0.48 | 248 | 4.99 | 0.68 | 255 | 4.86 |
Table 2. λMAX (nm), EMAX (eV), f of electronic transitions of L2F (n→π*) and its complexes and PdL2F (n→d) calculated using B3LYP, PBE0, CAM-B3LYP and PBE0 hybrid functional in gas, IEF-PCM and SS-PCM.
| Gas | IEF-PCM | SS-PCM | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λMAX | EMAX | f | λMAX | EMAX | f | λMAX | EMAX | f | λMAX | EMAX | |
| B3LYP | |||||||||||
| L2F | 337 | 3.68 | 0.14 | 327 | 3.80 | 0.18 | 332 | 3.73 | 0.23 | 327 | 3.79 |
| PdL2F | 366 | 3.38 | 0.08 | 352 | 3.52 | 0.08 | 356 | 3.48 | 0.13 | 410 | 3.02 |
| B3P86 | |||||||||||
| L2F | 339 | 3.66 | 0.13 | 331 | 3.75 | 0.18 | 336 | 3.69 | 0.22 | 327 | 3.79 |
| PdL2F | 362 | 3.43 | 0.08 | 407 | 3.05 | 0.06 | 411 | 3.02 | 0.12 | 410 | 3.02 |
| CAM-B3LYP | |||||||||||
| L2F | 301 | 4.12 | 0.20 | 293 | 4.23 | 0.22 | 299 | 4.15 | 0.27 | 327 | 3.79 |
| PdL2F | 365 | 3.40 | 0.16 | 346 | 3.59 | 0.21 | 353 | 3.51 | 0.33 | 410 | 3.02 |
| PBE0 | |||||||||||
| L2F | 325 | 3.81 | 0.17 | 318 | 3.90 | 0.19 | 324 | 3.83 | 0.24 | 327 | 3.79 |
| PdL2F | 347 | 3.57 | 0.08 | 387 | 3.20 | 0.09 | 392 | 3.16 | 0.17 | 410 | 3.02 |
Fig 3. UV-vis spectra of L2F and PdL2Fs.
Table 3. Calculated, scaled and experimental vibrational modes of L2F and its complexes PdL2F.
| L2F | PdL2F | |||||
|---|---|---|---|---|---|---|
| Cal | Scal | Exp | Cal | Scal | Exp | |
| νO-H | ||||||
| B3LYP | 2349 | 2274 | 2393 | - | - | - |
| B3P86 | 2092 | 2025 | 2393 | - | - | - |
| CAM-B3LYP | 2506 | 2426 | 2393 | - | - | - |
| PBE0 | 2214 | 2143 | 2393 | - | - | - |
| νC-H (ar) | ||||||
| B3LYP | 3250 | 3146 | 3274 | 3245 | 3141 | 3225 |
| B3P86 | 3269 | 3164 | 3274 | 3265 | 3160 | 3225 |
| CAM-B3LYP | 3277 | 3172 | 3274 | 3276 | 3171 | 3225 |
| PBE0 | 3280 | 3175 | 3274 | 3276 | 3171 | 3225 |
| νC = N | ||||||
| B3LYP | 1661 | 1608 | 1637 | 1643 | 1590 | 1616 |
| B3P86 | 1667 | 1613 | 1637 | 1662 | 1609 | 1616 |
| CAM-B3LYP | 1712 | 1657 | 1637 | 1695 | 1641 | 1616 |
| PBE0 | 1688 | 1634 | 1637 | 1677 | 1623 | 1616 |
| νC-H(sp2) | ||||||
| B3LYP | 3108 | 3008 | 3071 | 3139 | 3038 | 3041 |
| B3P86 | 3133 | 3032 | 3071 | 3155 | 3054 | 3041 |
| CAM-B3LYP | 3138 | 3037 | 3071 | 3170 | 3068 | 3041 |
| PBE0 | 3141 | 3040 | 3071 | 3251 | 3147 | 3041 |
| νC-H(sp3) | ||||||
| B3LYP | 3057 | 2959 | 2956 | 3037 | 2940 | 2960 |
| B3P86 | 3044 | 2946 | 2956 | 3051 | 2953 | 2960 |
| CAM-B3LYP | 3049 | 2951 | 2956 | 3061 | 2963 | 2960 |
| PBE0 | 3059 | 2961 | 2956 | 3061 | 2963 | 2960 |
Cal = Calculated; Scal = Scaled; Exp = Experimental
The strong maximum absorption band observed at the higher wavelength of 327 nm in the UV-Vis spectrum of L2F is assigned to n-π* electronic transition of the imine chromophore. This band indicates that there is a transition of electrons from the non-bonding n orbital to the anti-bonding π* orbital [47, 48]. Theoretically, this band corresponds to an electronic transition between HOMO and LUMO orbitals of L2F Schiff base. Referring to Table 2, in the gas phase, λMAX at 327 nm was overestimated with the B3LYP and B3P86 functionals and underestimated with CAM-B3LYP and PBE0 functionals. The best reproduction for the gas phase was obtained with the PBE0 hybrid functional with a variation of 2 nm with respect to the experimental value. This λMAX was seen to be strongly affected by the solvent with hypsochromic shifts (blue shift) obtained in different hybrid functionals. The exact reproduction of λMAX of L2F at 327 in IEF-PCM was obtained with B3LYP hybrid functional, while in SS-PCM the best reproduction was obtained with PBE0 hybrid functional with variation of 3 nm. The blue shift of λMAX at 327 nm in solvent is mainly referred to the solute-solvent interactions and to the formation of hydrogen bonding of L2F with solvent molecules. The results are in agreement with the values and trend reported by More et al., (2017) [49].
Upon complexation with Pd (II), the λMAX experienced a bathochromic (red) shift to a higher wavelength of 410 nm. This is in agreement with the weakening of the C = N when the lone pair of electrons on N is donated to the Pd (II) in a Lewis acid-base interaction. The best reproduction of this band was obtained with B3P86 in SS-PCM and IEF-PCM with variation of 1 and 3 nm with respect to the experimental value, respectively. It is worth to mention that the solute-solvent interactions induce a bathochromic shift (red shift) of the n-π* band.
Infrared spectroscopy
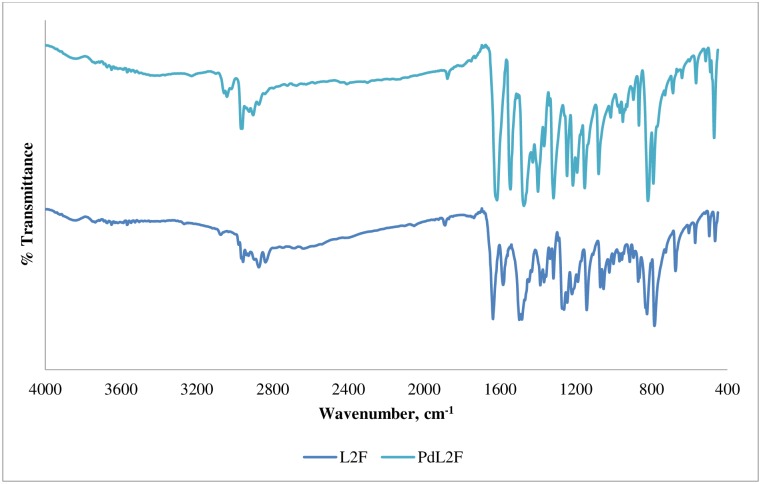
The calculated, scaled and experimental main vibrational modes of L2F and PdL2F are reported in Table 3. The characteristic peak for imine, ν(C = N), is found at 1637 cm-1 in the spectrum of the free ligand, L2F. The best reproduction of this band was obtained with PBE0 hybrid functional with a variation of 3 cm-1 with respect to the observed value (Table 3). Other tested functionals namely B3LYP, B3P86 and CAM-B3LYP, reproduced the peak less accurately with variations of 31, 24 and 20 cm-1 with respect to the observed value, respectively. This vibration mode experienced a shift of 21 cm-1 to a lower frequency of 1616 cm-1 in the spectrum of PdL2F as can be seen in Fig 4, indicating that complexation has been established through bonding of imine nitrogen and Pd (II) center [50]. The C = N bond became weaker upon complexation as a result of the inductive effect of lone electron pair on imine nitrogen being shared with the metal centre.
Fig 4. FTIR spectra of L2F and PdL2F.
The weak vibration of hydroxyl group ν(OH) of L2F is found at 2393 cm-1. The best reproduction of this vibration mode was obtained with B3LYP functional with a variation of 44 cm-1, while other tested functionals failed in its reproduction with variation higher than that. The weak peak of OH indicated the occurrence of hydrogen bonding OH···N = C occurring in intramolecular manner between –OH with imine nitrogen [51], as reported in Table 7. Noticeably, this signal disappeared in the PdL2F complex signalling that the complexation was established through deprotonation of the phenolic hydroxyl [52] prior to bonding with Pd (II) center. The shifting of ν(C-O) peak to a higher frequency by 8 cm-1 in PdL2F complex further supported the involvement of phenolic oxygen in complexation [53, 54].
Table 7. Hydrogen bonds in the ligand L2F and PdL2F complex.
| D --H ..A | D --H (Å) | H ..A (Å) | D --A (Å) | D --H ..A (°) |
|---|---|---|---|---|
| L2F | ||||
| O1 --H1B ..N1 | 0.83(2) | 1.86(3) | 2.619(2) | 152(3) |
| O2 --H2B ..N2 | 0.82(3) | 1.84(4) | 2.599(3) | 153(3) |
| PdL2F | ||||
| C5 --H5A ..O1 | 0.93 | 2.46 | 3.376(8) | 169 |
(symmetry codes; i = x,1/2-y,1/2+z)
NMR spectroscopy
The experimental and the predicted 1H and 13C chemical shifts of L2F and PdL2F are shown in Table 4 and Fig 5. The observed proton of OH group of L2F appears as singlet in the downfield region at 13.25 ppm due to the formation of intramolecular hydrogen bonding with the imine nitrogen [50]. Theoretically, this chemical shift was well reproduced in in gas and PCM phases, with variations of 0.45 and 0.25 ppm, respectively. The absence of OH peak in the spectra of PdL2F supported the infrared evidence that the coordination to metal centres was established through deprotonation of the hydroxyl groups [55]. The chemical shift for the imine proton, HC = N, was detected as a singlet in the region of 8.32 ppm for L2F. The predicted corresponding one in gas and PCM phases appeared at 7.9 and 8.0 ppm, respectively. The complexation of the ligand with Pd (II) shifted this peak downfield to 7.96 ppm. This shift was reproduced theoretically, appearing at 7.9 ppm. This supports the observation made in IR spectroscopy of the involvement of imine nitrogen in the coordination to the metal centre [56].
Table 4. Predicted and experimental 1H and 13C chemical shifts of L2F and its complex PdL2F.
| L2F | PdL2F | |||||
|---|---|---|---|---|---|---|
| 1H-NMR | Gas | PCM | Exp. | Gas | PCM | Exp. |
| OH | 12.8 | 13.5 | 13.25 | - | ||
| HC = N | 7.9 | 8.0 | 8.30 | 7.9 | 7.9 | 7.96 |
| Ar-H | 6.8 | 6.9 | 6.91–7.35 | 7.0 | 7.0 | 6.76–7.20 |
| CH3 | 1.5 | 1.3 | 0.97 | 1.2 | 1.1 | 1.02 |
| CH2 | 3.1 | 3.3 | 3.5 | 3.3 | 3.4 | 3.53 |
| 13C-NMR | ||||||
| C (OH) | 160 | 167 | 157.74 | - | ND | |
| HC = N | 164 | 172 | 164.74 | 164 | 163 | 164.4 |
| Ar-C | 113 | 121 | 116.35–119.51 | 115 | 116 | 117.76–120.66 |
| Ar-F | 157 | 164 | 156.34 | - | ND | |
| CH3 | 21 | 29 | 24.36 | 18 | 18 | 23.66 |
| -CH2- | 70 | 77 | 68.2 | 76 | 76 | 71.02 |
| -C(CH3)2 | 40 | 48 | 36.3 | 37 | 38 | 33.95 |
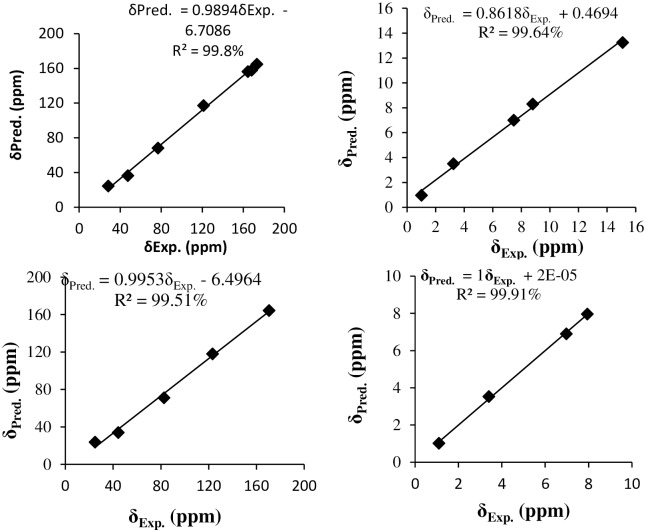
Fig 5. Correlation curves between the experimental and predicted 13C NMR (right) and 1H NMR (left) chemical shifts of L2F Schiff base (up) and its complex PdL2F (bottom).
The experimental and predicted chemical shifts of aromatic protons of free ligands appeared as multiplets in the range of 6.78–7.19 ppm for the free ligand. This is in agreement with the chemical shifts reported by Ahmad et al., (2018) [57]. These hydrogens experience the shielding effect of diamagnetic anisotropy caused by circulating π electrons in the aromatic rings [58]. The signals of these aromatic protons were found at 6.77–7.18 ppm in the complex. The shifting in chemical shift of these protons indicates that the complexation has been established between metal ions and the ligands. Good correlations are obtained between the experimental and the predicted proton chemical shifts of L2F and its complex PdL2F with correlation coefficient coefficients of 99.64 and 99.91%, respectively.
The 13C NMR chemical shift of azomethine carbon, HC = N is found at 164.74 ppm in the free ligand. The corresponding predict one appears at 164 ppm in gas phase. However, in PCM phase downfield of the signal is observed with a variation of 7.26 ppm with respect to the experimental value. The signal of C-OH is observed at 158 ppm, slightly up field with respect to HC = N signal. Characteristic peaks of aliphatic and aromatic methyl were observed at 36.30 ppm and 24.36 ppm, respectively. Aromatic carbons were detected in the range of 117.96–156.34 ppm. The 13C NMR chemical shifts of L2F and PdL2F are well reproduced with correlation coefficients of 99.8 and 99.51%, respectively (Fig 5).
Crystal structure description
The ligand L2F and complex PdL2F crystallized in Pī and monoclinic system respectively. The crystal system and refinement parameters are given in Table 5.
Table 5. Crystallographic data and refinement parameters for L2F and PdL2F.
| Compound | L2F | PdL2F |
|---|---|---|
| Chemical formula | C19H20F2N2O2 | C19H18F2N2O2Pd |
| Molecular weight | 346.37 | 450.75 |
| Temperature (K) | 0(2) | 273(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Triclinic | monoclinic |
| Space group | Pī | P21/c |
| Unit cell dimensions (Å) | a = 6.1334(5), b = 9.2490(7), c = 16.3270(13) | a = 11.111(2), b = 13.701(3), c = 12.196(2) |
| Angles(°) | α = 102.110(2), β = 96.032(2), γ = 103.520(2) | β = 105.069(6) |
| Volume (Å3) | 868.84(12) | 1792.8(6) |
| Z | 2 | 4 |
| Absorption coefficient (mm-1) | 0.101 | 1.313 |
| F(0 0 0) | 364 | 904 |
| Crystal size | 0.50 × 0.29 × 0.19 mm | 0.27 × 0.20 × 0.08 mm |
| No. of reflections collected | 25298 | 51046 |
| No. of independent reflections | 4327 | 4443 |
| θ range (°) | 2.930–28.370 | 2.973–28.353 |
| Index ranges | -7< = h< = 8 | -14< = h< = 14 |
| -12< = k< = 12 | -18< = k< = 18 | |
| -21< = l< = 21 | -16< = l< = 16 | |
| Goodness-of-fit on F2 | 1.052 | 1.106 |
| R indices | R1 = 0.0626, wR2 = 0.1294 | R1 = 0.0646, wR2 = 0.1229 |
| R indices (all data) | R1 = 0.1003, wR2 = 0.1500 | R1 = 0.0925, wR2 = 0.1428 |
| CCDC no. | 1855625 | 1890435 |
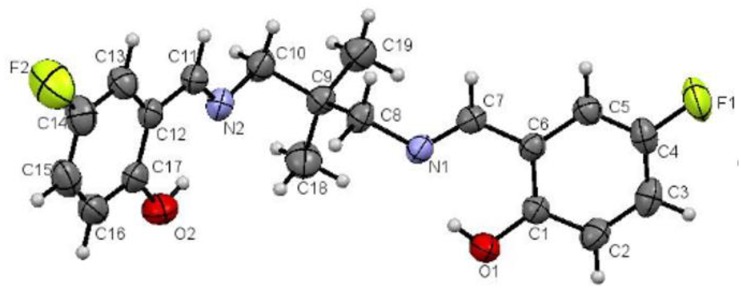
Fig 6 shows the molecular structure of the ligand with the numbering scheme. The molecule consists of two 2-fluoro (2-iminoethyl) phenol groups linked by 2,2-dimethyl-1,3- dimethylenepropane moiety.
Fig 6. Molecular structure of ligand drawn at 50% probability ellipsoids.
The two 2-fluoro (2-iminoethyl) phenol groups F1/O1/N1/C1-C8 and F2/O2/N1/C10-C17 are each planar with maximum deviation of 0.039(2) and 0.048(2) Å for fluorine atom (F1 and F2) respectively from their mean square planes. Both planes make dihedral angle of 42.26°. The N1/C8/C9 and N2/C10/C9 are planar with maximum deviation of 0.008 (2) Å for atom N1 from the least square plane and the torsion angle of C9-C8-N1-C7 and C9-C10-N2-C11 are 147.68 ° and 138.19(19) ° respectively. The bond length and angles are in normal ranges (Table 6). There are two intramolecular hydrogen bonds O1-H1A…N1 and O2-H2A…N2 in the molecule (Table 7). No intermolecular hydrogen bonds were observed.
Table 6. Some important bond lengths (Å) and angles (°) of L2F and PdL2F.
| Bond length/angle | L2F | PdL2F |
|---|---|---|
| O1-C1 | 1.348(2) | 1.306(7) |
| O2-C17 | 1.349(3) | 1.308(7) |
| N1-C7 | 1.270(2) | 1.280(8) |
| N1-C8 | 1.458(2) | 1.480(7) |
| N2-C11 | 1.270(3) | 1.297(8) |
| N2-C10 | 1.456(3) | 1.467(7) |
| Pd1-O1 | - | 1.994(4) |
| Pd1-N2 | - | 1.997(5) |
| Pd1-O2 | - | 2.000(4) |
| Pd1-N1 | - | 2.013(4) |
| O1-Pd1-N2 | - | 172.85(18) |
| O1-Pd1-O2 | - | 80.42(17) |
| N2-Pd1-O2 | - | 92.49(18) |
| O1-Pd1-N1 | - | 92.33(18) |
| N2-Pd1-N1 | - | 94.79(19) |
| O2-Pd1-N1 | - | 172.33(18) |
| C8-C9-C10-N2 | - | -73.8(7) |
| C19-C9-C10-N2 | - | 170.5(5) |
| C18-C9-C10-N2 | - | 50.4(7) |
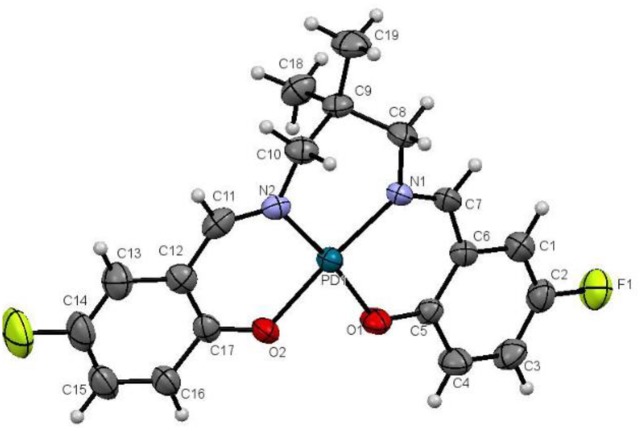
The ligand formed 1:1 complex with palladium acetate through coordination via nitrogen and phenolic oxygen atoms to form a square planar geometry (Fig 7). Both phenolic OH groups were deprotonated. The nitrogen atoms are at trans position to oxygen atoms. The trans N-Pd1-O angles are about 172° and the cis angles about the central Pd1 atom between 80.42 (17) and 94.79 (19)° indicate a distorted square planar geometry.
Fig 7. Molecular structure of PdL2F complex drawn at 50% probability ellipsoids.
The coordination also creates three 6-membered rings, Pd1-N1-C7-C8-C5-O1, Pd1-N2-C11-C12-C17-O2 and Pd1-N1-C8-C9-C10-N2. The first two rings are essentially planar with maximum deviation of 0.079 (4) Å for O2 atom from the least square plane. However, the Pd1-N1-C8-C9-C10-N2 ring adopts half chair conformation with C9 atom deviated by 0.404(6) Å for C10 atom from the least square plane (Fig 7).
The O1-C1 and O2-C17 bond lengths in the complex are significantly shorter than that in the ligand as the result of the deprotonation of the phenolic groups. On the other hand, the N1-C7 and N2-C11 bond lengths are slightly longer than that in the ligand. Other bond lengths and angles are in normal ranges and comparable with those reported by Ahmad et al., (2017) [59].
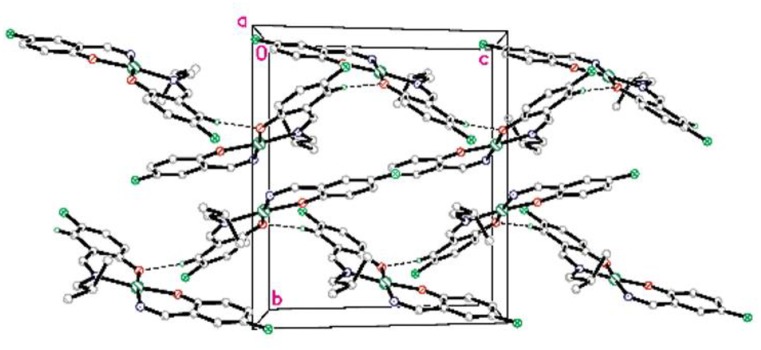
In the crystal structure the complex is stabilized by C5-H5A…O1 intermolecular hydrogen bonds (symmetry code as in Table 7) to a zig-zag one dimensional chain along the c axis (Fig 8).
Fig 8. Molecular packing of PdL2F complex viewed down a axis.
The non-hydrogenbonded hydrogen atoms are omitted for clarity.
Biological activity screening
L2F and PdL2F were screened for their anticancer properties against HCT116. As depicted in Table 8, PdL2F showed a better potency against the cancer cell with IC50 of 4.1 μg/mL while L2F could be considered less active as anticancer agent with 90.00 μg/mL. Located in the same group as platinum, as well as being a soft metal with an abundance of d electrons, palladium is expected to interact with the DNA backbone that is rich with N, a soft donor atom. The excellent performance of the complex may also be caused by its square planar geometry which allowed them to stack in the structure of the DNA [60]. The structure helped palladium (II) complex kinking the pharmacological target, DNA, more effectively through a mechanism called intercalation [61]. Intercalation has been proven to stabilize, lengthen, stiffen, and at certain extent unwinds the DNA double helix. Intercalators can fit between two base pairs, opening a space between its base pairs by unwinding [62].
Table 8. The anticancer activity of L2F and its PdL2F complex against HCT116.
| Compound | IC50, μg/mL |
|---|---|
| L2F | 90.00 (less active) |
| PdL2F | 4.1 (active) |
| Positive control (5-FU) | 1.70 |
| Vehicle Control (DMSO)* | 1.09 |
Conclusion
2,2'-((1E,1'E)-((2,2-dimethylpropane-1,3-diyl)-bis(azanylylidene))bis(methanylylidene))bis(4-fluorophenol), a tetradentate Schiff base named L2F and its complex PdL2F, were successfully synthesised. The molecular structures of the synthesised compounds were confirmed by spectroscopic and X-ray techniques. Anticancer activity of PdLF was better than its parent ligand against HCT1116 with 1C50 of 4.1 μg/mL.
Supporting information
(DOCX)
(DOCX)
(CIF)
(CIF)
Acknowledgments
The authors would like to thank Coordination Chemistry Laboratory, Atta-ur-Rahman Institute for Natural Product Discovery (AuRIns), Collaborative Drug Discovery Research (CDDR) Group and Universiti Teknologi MARA (UiTM) for all the facilities used to conduct this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author received funding from research funding (600-IRMI/5/3 LESTARI (024/2019)) and the Ministry of Higher Education of Malaysia for SLAB/SLAI scholarship.
References
- 1.Tidwell T. T. Hugo (Ugo) Schiff, Schiff Bases, and a century of β-Lactam synthesis. Angewandte Chemie. 2008: 1016–1020. 10.1002/anie.200702965 [DOI] [PubMed] [Google Scholar]
- 2.Gupta K. C., & Sutar A. K. Catalytic activities of Schiff base transition metal complexes. Coordination Chemistry Reviews. 2008; 252: 1420–1450. [Google Scholar]
- 3.Baleizao C., & Garcia H. Chiral salen complexes: an overview to recoverable and reusable homogeneous and heterogeneous catalysts. Chem. Rev., 2006;106: 3987–4043. 10.1021/cr050973n [DOI] [PubMed] [Google Scholar]
- 4.Keypour H., Salehzadeh S., & Parish R. V. Synthesis of two potentially heptadentate (N4O3) Schiff-base ligands derived from condensation of tris(3-aminopropyl)-amine and salicylaldehyde or 4-hydroxysalicylaldehyde. Nickel(II) and copper(II) complexes of the former ligand. Molecules. 2002; 7(2):140–144. [Google Scholar]
- 5.Abdel-Rahman L. H., Abu-Dief A. M., El-Khatib R. M., & Abdel-Fatah S. M. Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorganic Chemistry. 2016; 69: 140–152. 10.1016/j.bioorg.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 6.Alim A., Zahan K. E., Haque M., & Tarafder M. Synthesis and characterization of some metal complexes of Cu(II), Ni(II), Zn(II), Cd(II), Sn(II), Co(II), Sb(III) and Fe(III) containing bidentate Schiff base of Smdtc. Science Journal of Chemistry. 2015; 3(3): 35–39. [Google Scholar]
- 7.Tarafder M., Kasbollah A., Saravanan N., Crouse K.A., Ali A.M., S-methyldithiocarbazate and its Schiff bases: evaluation of bondings and biological properties. Journal of Biochemistry, Molecular Biology, and Biophysics: JBMBB: the official journal of the Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB). 2002; 6(2): 85. [DOI] [PubMed] [Google Scholar]
- 8.Chohan Z.H., Arif M., Shafiq Z., Yaqub M., Supuran C.T., In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt(II), copper (II), nickel (II) and zinc (II) complexes, Journal of Enzyme Inhibition and Medicinal Chemistry. 2006; 21(1): 95–103. 10.1080/14756360500456806 [DOI] [PubMed] [Google Scholar]
- 9.Guo Z., Xing R., Liu S., Zhong Z., Ji X., Wang L., et al. , Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydrate Research. 2007; 342(10): 1329–1332. 10.1016/j.carres.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Taha M., Baharudin M.S., Ismail N.H., Khan K.M., Jaafar F.M., Siddiqui S., et al. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorganic & Medicinal Chemistry Letters. 2013; 23(11): 3463–3466 [DOI] [PubMed] [Google Scholar]
- 11.Anouar E.H., Raweh S., Bayach I., Taha M., Baharudin M.S., Di Meo F., et al. Trouillas, Antioxidant properties of phenolic Schiff bases: structure–activity relationship and mechanism of action. Journal of Computer-aided Molecular Design. 2013; 27(11): 951–964. 10.1007/s10822-013-9692-0 [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimipour S.Y., Abaszadeh M., Castro J., Seifi M., Synthesis, X-ray crystal structure, DFT calculation and catalytic activity of two new oxido-vanadium(V) complexes containing ONO tridentate Schiff bases. Polyhedron. 2014; 79: 138–150. [Google Scholar]
- 13.Tajuddin A.M., Ramasamy K., Yamin B.M., Alharthi A.I., Bahron H., DFT analysis and bioactivity of 2-((E)-(4-methoxybenzylimino)methyl) phenol and its Ni(II) and Pd(II) complexes. Arabian Journal of Chemistry. 2017; 10(6): 769–780. [Google Scholar]
- 14.Gauss J., Calculation of NMR chemical shifts at second-order many-body perturbation theory using gauge-including atomic orbitals. Chemical Physics Letters. 1992;191(6): 614–620. [Google Scholar]
- 15.Gauss J. Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts, J. Chem. Phys. 1993; 99(5): 3629–43. [Google Scholar]
- 16.Gauss J. Accurate calculation of NMR chemical shifts, Ber. Bunsen-Ges. 1995;99(8):1001–1008. [Google Scholar]
- 17.Bak K., Hansen A., Ruud K., Helgaker T., Olsen J., Jørgensen P. Ab initio calculation of electronic circular dichroism fortrans-cyclooctene using London atomic orbitals, Theoret. Chim. Acta. 1995:90(5–6): 441–458. [Google Scholar]
- 18.Bauernschmitt R., Ahlrichs R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chemical Physics Letters. (1996); 256(4–5): 454–464. [Google Scholar]
- 19.Casida M.E., Jamorski C., Casida K.C., Salahub D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: characterization and correction of the time-dependent local density approximation ionization threshold. The Journal of Chemical Physics. 1998;108(11): 4439–4449. [Google Scholar]
- 20.Mendoza-Wilson A.M., Glossman-Mitnik D. CHIH-DFT study of the electronic properties and chemical reactivity of quercetin. Journal of Molecular Structure. THEOCHEM. 2005; 716(1–3): 67–72. [Google Scholar]
- 21.Vázquez-Vuelvas O.F., Hernández-Madrigal J.V., Gaviño R., Tlenkopatchev M.A., Morales-Morales D., Germán-Acacio J.M., et al. X-ray, DFT, FTIR and NMR structural study of 2,3-dihydro-2-(R-phenylacylidene)-1,3,3-trimethyl-1H-indole. Journal of Molecular Structure. 2011; 987(1–3):106–118. [Google Scholar]
- 22.Jacquemin D., Perpete E.A., Scuseria G.E., Ciofini I., Adamo C. TD-DFT performance for the visible absorption spectra of organic dyes: conventional versus long-range hybrids. Journal of Chemical Theory and Computation. 2007; 4(1): 123–135. [DOI] [PubMed] [Google Scholar]
- 23.Jacquemin D., Preat J., Charlot M., Wathelet V., Andre J.-M., Perpete E.A. Theoretical investigation of substituted anthraquinone dyes. The Journal of Chemical Physics. 2004; 121(4): 1736–1743. 10.1063/1.1764497 [DOI] [PubMed] [Google Scholar]
- 24.Jacquemin D., Preat J., Wathelet V., Fontaine M., Perpète E.A. Thioindigo dyes: highly accurate visible spectra with TD-DFT. Journal of the American Chemical Society. 2006; 128(6): 2072–2083. 10.1021/ja056676h [DOI] [PubMed] [Google Scholar]
- 25.Jacquemin D., Wathelet V., Preat J., Perpète E.A. Ab initio tools for the accurate prediction of the visible spectra of anthraquinones. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2007; 67(2): 334–341. 10.1016/j.saa.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 26.Woodford J.N. A DFT investigation of anthocyanidins. Chemical Physics Letters. 2005; 410(4–6): 182–187. [Google Scholar]
- 27.Anouar E.H., Gierschner J., Duroux J.-L., Trouillas P., UV/Visible spectra of natural polyphenols: A time-dependent density functional theory study. Food Chemistry. 2012; 131(1):79–89. [Google Scholar]
- 28.Lumpi D., Horkel E., Plasser F., Lischka H., Fröhlich J. Synthesis, spectroscopy, and computational analysis of photoluminescent bis(aminophenyl)‐substituted thiophene derivatives. ChemPhysChem. 2013;14(5): 1016–1024. 10.1002/cphc.201201006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quartarolo A.D., Russo N. A computational study (TDDFT and RICC2) of the electronic spectra of pyranoanthocyanins in the gas phase and solution. Journal of Chemical Theory and Computation. 2011; 7(4): 1073–1081. 10.1021/ct2000974 [DOI] [PubMed] [Google Scholar]
- 30.Ramos Sousa F.F., Quartarolo A.D., Sicilia E., Russo N. A time-dependent density functional study of a non-aromatic [1.1. 1.1. 1]-pentaphyrin and its lutetium complex. The Journal of Physical Chemistry B. 2012; 116(35): 10816–10823. 10.1021/jp3068359 [DOI] [PubMed] [Google Scholar]
- 31.Wolinski K., Hinton J.F., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. Journal of the American Chemical Society. 1990; 112(23): 8251–8260. [Google Scholar]
- 32.Cheeseman J.R., Trucks G.W., Keith T.A., Frisch M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. The Journal of Chemical Physics. 1996; 104: 5497. [Google Scholar]
- 33.Data. Germany: University of Gottingen; 1996.
- 34.Sheldrick GM. SHELXTL V5.1, Software Reference Manual. Madison, WI, USA: Brukel AXS Inc; 1997. [Google Scholar]
- 35.Rohilla P., Deep A., Kamra M., Narasimhan B., Ramasamy K., Mani V., … et al. Antimicrobial and Anticancer Evaluation of N ′- (Substituted Benzylidene)-2-(Benzo[d]oxazol-3(2H) -yl) acetohydrazide Derivatives. Drug Research. 2014; 13, 1–5. [DOI] [PubMed] [Google Scholar]
- 36.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. Journal of Chemical Physics. 1993; 98(7): 5648–52. [Google Scholar]
- 37.Andersson M., Uvdal P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6–311+ G (d, p). The Journal of Physical Chemistry A. 2005; 109(12): 2937–2941. 10.1021/jp045733a [DOI] [PubMed] [Google Scholar]
- 38.Furche F., Ahlrichs R. Adiabatic time-dependent density functional methods for excite d state properties. The Journal of Chemical Physics. 2002; 117: 7433. [Google Scholar]
- 39.Scalmani G., Frisch M.J., Mennucci B., Tomasi J., Cammi R., Barone V. Geometries and properties of excited states in the gas phase and in solution: theory and application of a time-dependent density functional theory polarizable continuum model. The Journal of Chemical Physics. 2006; 124: 094107. [DOI] [PubMed] [Google Scholar]
- 40.Gauss J. Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts. J. Chem. Phys. 1993; 99: 3629–43. [Google Scholar]
- 41.Tomasi J., Mennucci B., Cammi R. Quantum mechanical continuum solvation models. Chem. Rev. 2005; 105(8): 2999–3093. 10.1021/cr9904009 [DOI] [PubMed] [Google Scholar]
- 42.Jacquemin D., Wathelet V., Perpete E.A., Adamo C. Extensive TD-DFT benchmark: singlet-excited states of organic molecules. Journal of Chemical Theory and Computation. 2009; 5(9): 2420–2435. 10.1021/ct900298e [DOI] [PubMed] [Google Scholar]
- 43.Improta R., Barone V., Scalmani G., Frisch M.J. A state-specific polarizable continuum model time dependent density functional theory method for excited state calculations in solution. The Journal of Chemical Physics. 2006; 125: 054103 10.1063/1.2222364 [DOI] [PubMed] [Google Scholar]
- 44.Improta R., Scalmani G., Frisch M.J., Barone V. Toward effective and reliable fluorescence energies in solution by a new state specific polarizable continuum model time dependent density functional theory approach. The Journal of Chemical Physics. 2007; 127: 074504 10.1063/1.2757168 [DOI] [PubMed] [Google Scholar]
- 45.G.W.T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, eet al. Gaussian 09, Revision A.02, 2009.
- 46.Khanmohammadi H., Salehifard M., & Abnosi M. H. Synthesis, characterization, biological and thermal studies of Cu(II) complexes of salen and tetrahedrosalen ligands. Journal of the Iranian Chemical Society. 2009; 6(2): 300–309. [Google Scholar]
- 47.Bosnich B. An interpretation of the circular dichroism and electronic spectra of salicylaldimine complexes of square-coplanar diamagnetic nickel(II). Journal of the American Chemical Society. 1968; 90(3): 627–632. [Google Scholar]
- 48.Senol C., Hayvali Z., Dal H., & Hokelek T. Syntheses, characterizations and structures of NO donor Schiff base ligands and nickel(II) and copper(II) complexes. Journal of Molecular Structure. 2011; 997: 53–59. [Google Scholar]
- 49.More M. S., Pawal S. B., Lolage S. R., & Chavan S. S. Syntheses, structural characterization, luminescence and optical studies of Ni(II) and Zn(II) complexes containing salophen ligand. Journal of Molecular Structure. 2017; 1128, 419–427. [Google Scholar]
- 50.Nair M. S., Arish D., & Johnson J. Synthesis, characterization and biological studies on some metal complexes with Schiff base ligand containing pyrazolone moiety. Journal of Saudi Chemical Society. 2016; 20: S591–S598. [Google Scholar]
- 51.Aranha P. E., Santos M. P., Romera S., & Dockal E. R. (2006). Synthesis, characterization, and spectroscopic studies of tetradentate Schiff base chromium (III) complexes. Polyhedron. 2006; 26(7): 1373–1382. [Google Scholar]
- 52.Raman N., Kulandaisamy A., Thangaraja C., Manisankar P., Viswanathan S., & Vedhi C. Synthesis, structural characterisation and electrochemical and antibacterial studies of Schiff base copper complexes. Transition Metal Chemistry. 2004; 29(2): 129–135. [Google Scholar]
- 53.Hille A., Ott I., Kitanovic A., Kitanovic I., Alborzinia H., Lederer E., … et al. [N,N′-Bis(salicylidene)-1,2-phenylenediamine]metal complexes with cell death promoting properties. Journal of Biological Inorganic Chemistry. 2009; 14(5), 711–725. 10.1007/s00775-009-0485-9 [DOI] [PubMed] [Google Scholar]
- 54.Kolawole G. A., & Patel K. S. The stereochemistry of oxovanadium(IV) complexes derived from salicylaldehyde and polymethylenediamines. Journal of the Chemical Society, Dalton Transactions. 1981; 6: 1241–1444. [Google Scholar]
- 55.Raman N., Pitchaikani Raja Y., & Kulandaisamy A. Synthesis and characterisation of Cu(II), Ni(II), Mn(II), Zn(II) and VO(II) Schiff base complexes derived from o-phenylenediamine and acetoacetanilide. Chem. Sci. 2001; 113(3): 183–189. [Google Scholar]
- 56.Maity D., Drew M. G.., Godsell J. F., Roy S., & Mukhopadhyay G. Synthesis and characterization of Cu (II) complexes of tetradentate and tridentate symmetrical Schiff base ligands. Transition Metal Chemistry. 2010; 35: 197–204. [Google Scholar]
- 57.Ahmad S. N., Bahron H., & Tajuddin A. M. Tetradentate palladium(II) salophen complexes: synthesis, characterization and catalytic activities in copper-free Sonogashira coupling reaction. International Journal of Engineering & Technology. 2018; 7(3.11): 15–19. [Google Scholar]
- 58.M.Lampman, G., L.Pavia, D., S.Kriz, G., & R.Vyvyan, J. Spectroscopy. 4th ed. 2010
- 59.Ahmad S. N., Bahron H., Tajuddin A. M., & Yusof M. S. M. Crystal structure of 2,2′-(((2,2-Dimethylpropane-1,3-diyl)bis(azanylylidene))-bis(methanylylidene))bis(4-methoxyphenol)palladium(II). X-Ray Structure Analysis Online. 2017; 33: 73–74. [Google Scholar]
- 60.Arola-arnal A., Benet-buchholz J., & Neidle S. Effects of metal coordination geometry on stabilization of human telomeric quadruplex DNA by square-planar and square-pyramidal metal complexes. Inorganic Chemistry. 2008; 47(24): 11910–11919. 10.1021/ic8016547 [DOI] [PubMed] [Google Scholar]
- 61.Liu H.-K., & Sadler P. J. Metal Complexes as DNA Intercalators. Accounts of Chemical Research. 2011; 44(5): 349–359. 10.1021/ar100140e [DOI] [PubMed] [Google Scholar]
- 62.Sirajuddin M., & Ali S. Drug—DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry spectroscopies and cyclic voltametry. Journal of Photochemistry and Photobiology A: Chemistry. 2013; 124: 1–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(CIF)
(CIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.