Scheme 1. Two possible mechanisms of C-terminal cross-link formation from an Asn residue.
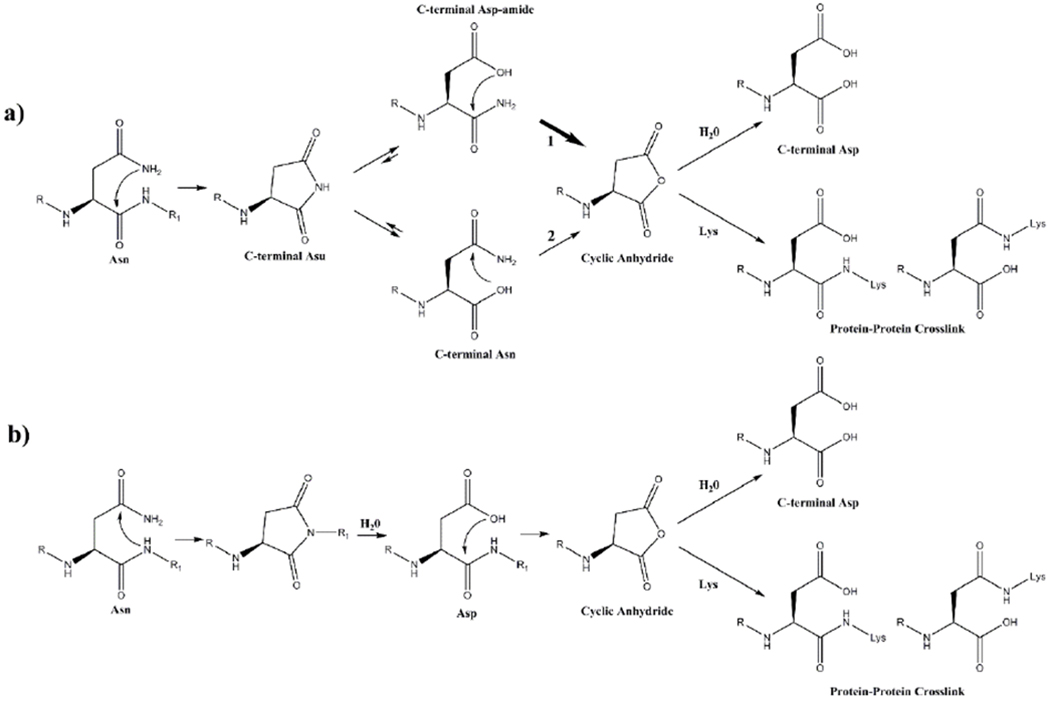
a) Spontaneous cleavage at an Asn residue leads to the formation of a C-terminal succinimide (Asu). This succinimide hydrolyses to yield both a C-terminal Asn and a C-terminal Asp amide. Each of these can deamidate to form a cyclic succinic anhydride. This intermediate can either hydrolyse to Asp/ isoAsp or, in the presence of a nucleophile such as Lys, can form a protein-protein crosslink.
b) Prior to peptide bond cleavage, deamidation of Asn occurs. The newly formed Asp, can then undergo spontaneous cyclisation forming a succinic anhydride intermediate. The cyclic anhydride can react with an amine group, such as that of a Lys residue, forming an Asp-Lys crosslink. The ε-amino group of Lys can potentially attack either carbonyl of the anhydride leading to two isomeric Asp-Lys crosslinks as shown.
