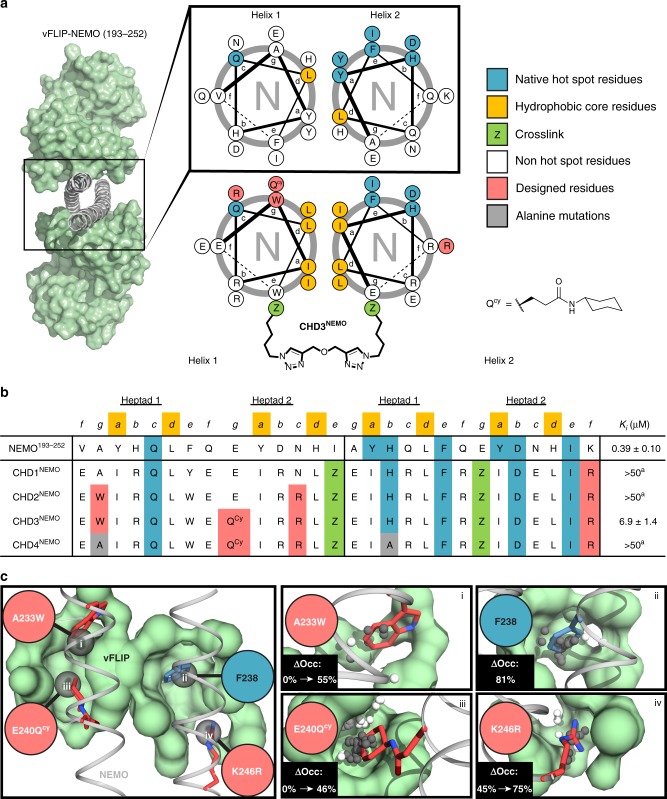
Fig. 2. Rational design of inhibitors of the NEMO-vFLIP interaction.
a Helical wheel diagram depicting native (top) NEMO coiled coil and optimized (bottom) sequences. A crosslinker is placed at the e and g positions to constrain the dimer. b Peptide sequences of designed compounds and their respective inhibitory constants from the TR-FRET assay. Superscript ‘a’ denotes incomplete dissociation of the NEMO-vFLIP complex observed at 100 μM concentrations (Fig. 3a). c Cartoon depicts four high-ranking pockets identified by AlphaSpace and corresponding residues from CHD3NEMO. The changes in %pocket occupancy as a result of three mutations are highlighted.