Fig. 5. Model for the metabolic adaptations that ameliorate NOX2 killing of intracellular Salmonella.
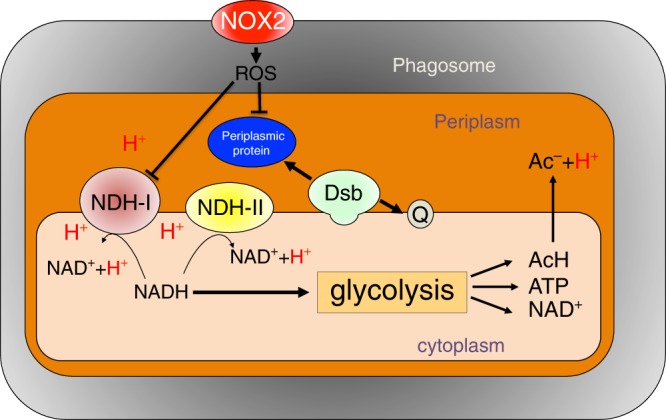
Reactive oxygen species (ROS) produced by NOX2 in the phagosome target the proton-coupled NDH-I isoform of NADH dehydrogenase of Salmonella. Oxidative stress also diminishes transcription of nuo genes encoding NDH-I. Together, the negative effects oxidative stress has on the expression of NDH-I contributes to the dissipation of ΔpH across the bacterial membrane. Collapse of ΔpH negatively impacts the function of the oxidoreductase Dsb system, which introduces disulfide bonds among in cysteine residues of periplasmic proteins (blue). Collapse of ΔpH, defective Dsb function and oxidation of cell envelope proteins catastrophically synergize to mediate NOX2-dependent killing of intracellular Salmonella. In response to the selective pressure oxidative stress imposes on the ETC, Salmonella shuttles of NADH reducing power to fermentation, thereby not only diminishing electron flow through ETC but also allowing for proper folding of periplasmic proteins by Dsb oxidoreductases that delivered the extracted reducing power into the quinones (Q) of the ETC. Fermentation of glucose also provides ATP synthesis by substrate-level phosphorylation, balances NAD+/NADH, and helps maintain ΔpH across membrane via dissociation of products such as acetic acid (AcH).
