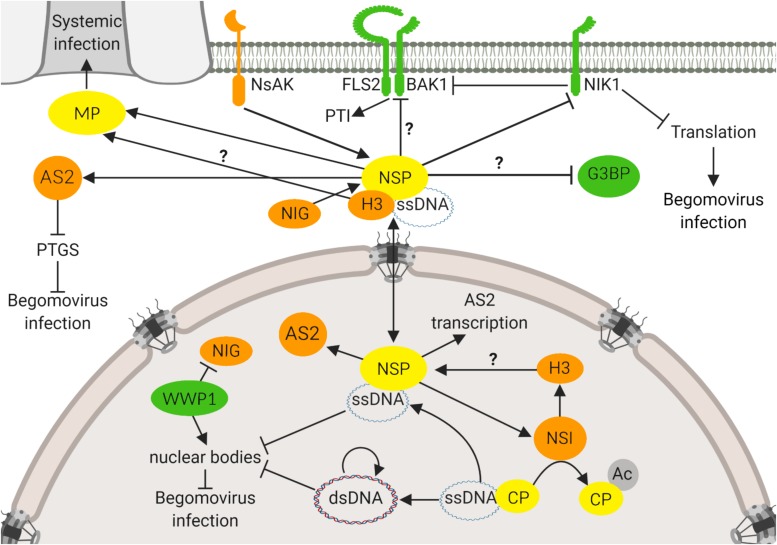
FIGURE 1.
NSP-interacting immune hub. The NSP-interacting immune hub consists of host proteins displaying antiviral functions (green) and proviral functions (orange). Viral proteins are indicated in yellow. In the nucleus, CaLCuV NSP binds to AtNSI to facilitate the acetylation of CP that favors NSP-vDNA complex formation. BDMV NSP also binds to H3. CaLCuV NSP increases the expression of proviral AS2, an inhibitor of PTGS, and also promotes its reallocation to the cytosol. At the cytosolic, perinuclear region, CaLCuV NSP interacts with NIG to facilitate the release of the vDNA-NSP complex from the nuclear pore into the cytosol. To prevent the NIG proviral function, the antiviral protein WWP1 sequesters NIG into nuclear bodies (NB), impairing its cytosolic transport function. As a counter defensive measure, vDNA disrupts the WWP1-NB assembly. At the cytoplasm, NSP may interact with G3BP, a protein required for the assembly of stress granules. NSP also interacts with the viral MP, which may provide the directionality for the NSP-vDNA-H3 complex toward the plasmodesmata for translocation to the neighboring cells. At the plasma membrane, NSP interacts with the receptor-like kinase NIK1 to suppress its kinase activity that otherwise would transduce an antiviral signal against begomoviruses. NIK1 also inhibits PTI, via interaction with BAK1 that may also be a target of the suppressing activity of NSP. NSP may serve as a substrate for another receptor-like kinase, NsAK, at the plasma membrane. Question marks denote not fully characterized proviral or antiviral functions of host proteins. Arrows indicate a positive effect on protein activity, and inhibitor lines denote suppressing activities. Double arrow indicates the double direction of protein movement. The figure was created with BioRender.com.