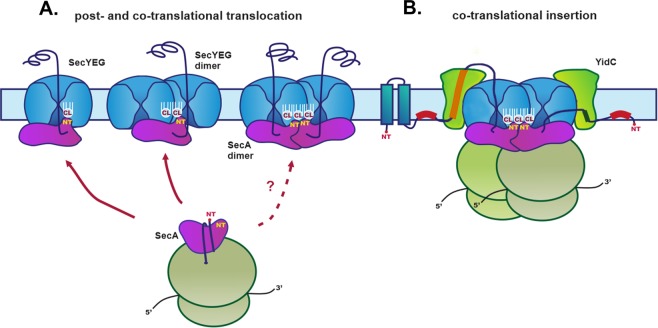
Figure 5.
Translocon and CL mediated protein co- and post-translational membrane translocation and co-translational insertion of proteins in E. coli. (A) Proteins are delivered to the SecYEG translocon by SecA-dependent pathway utilized by secreted periplasmic and OM proteins. Although a single copy of SecY is sufficient for simultaneous binding to SecA and translocating a substrate (left), a second SecYEG molecule maybe required for efficient SecA binding26. A scenario in which SecA would still bind to one copy of SecYEG but translocation would proceed through the a second SecYEG is shown (second from left). The functional oligomeric state of SecA during the protein translocation cycle is still a controversial issue. Both monomeric and dimeric forms have been suggested to be physiologically relevant. The SecA monomers (or dimers, dashed line) engage transiently with the SecYEG. The SecA monomers can co-translationally recognizes the nascent chain of an IM protein with high affinity and specificity7. (B) During lateral discharge from the dimerized SecYEG channel, a nascent IM protein interacts with YidC, an intramembrane insertase which assists in sidewise release of membrane proteins. The stability of the dimeric SecYEG translocon and its association with SecA is altered in CL-depleted cells (Fig. 4) indicating that the lack of CL destabilizes these interactions, thus affecting all possible scenarios shown in the diagram. Efficient binding of SecA to a translocating SecY channel requires that SecA first interacts through its N-terminus with negatively charged lipids in the membrane10, which enriches SecA in the vicinity of SecYEG. SecA drives translocation through the SecYEG channel by ATP hydrolysis. When SecA is lost from SecY in its ADP-bound state, the polypeptide chain is no longer pushed into the channel11. In the presence of dianionic CL, the affinity of SecA for the membrane and/or SecYEG is stronger and SecA is more frequently associates with the SecY channel, thereby indirectly accelerating the translocation process. Thus, CL in the vicinity of SecYEG dimer can contribute to appropriate timing and efficiency of SecA cycling between high- and low-affinity states determined by the equilibrium between ADP- and ATP bound states.