Abstract
Background
Treatment of subarachnoid neurocysticercosis (NCC) is complicated, and assays that can guide treatment are not widely available. The reproducibility and scalability of molecular-based biomarkers would be of great use.
Methods
The Taenia solium genome was mined and primers and probes were designed to target repeats with the highest coverage; the most sensitive, specific, and efficient repeat (TsolR13) was selected for clinical testing. We tested 46 plasma samples and 36 cerebral spinal fluid (CSF) samples taken from patients with subarachnoid or ventricular disease using quantitative polymerase chain reaction (qPCR).
Results
The analytic sensitivity of TsolR13 was 97.3% at 240 attograms (ag) of T. solium genomic DNA and 100% analytic specificity. The clinical sensitivity in detecting active subarachnoid or ventricular disease in symptomatic patients was 100% in CSF and 81.3% in plasma. The predictive ability to distinguish active from cured disease was better for CSF (94.4% of those cured had negative qPCR results) than for plasma (86.7% of those cured tested negative). Some subjects also had plasma DNA detectable intermittently for years after being cured. Overall, the test performance was equivalent to T. solium antigen detection.
Conclusions
A qPCR test for the detection of the highly repetitive Tsol13 sequence has been developed and shown to be highly sensitive and specific for NCC, but also useful as a test of cure in CSF and for the definitive diagnosis of NCC in plasma.
Keywords: PCR, Taenia solium, neurocysticercosis, subarachnoid disease, biomarker
A new quantitative polymerase chain reaction target, TsolR13, which is highly specific in diagnosing neurocysticercosis when testing plasma and when assessing cerebral spinal fluid, is also a useful biomarker for diagnosing and monitoring patients with subarachnoid neurocysticercosis.
(See the Editorial Commentary by White Jr and Coyle on pages 1882–3.)
The global burden of neurocysticercosis (NCC) is conservatively estimated at 2.8 million Disability Adjusted Life Years due to seizures caused by cysts found within the brain substance (parenchymal disease) [1]. Not considered in this number is the impact of disabilities caused by the most severe forms of the disease, when cysts are found within the ventricles (ventricular disease) or proliferate in the basilar cisterns of the brain (“racemose” subarachnoid disease, here referred to as SANCC). These patients often require the placement of a ventriculoperitoneal shunt for hydrocephalus [2, 3] and/or brain surgery for cyst removal, as well as months to years of anthelmintic therapy with concomitant suppression of the exuberant inflammatory response [4].
The diagnosis of SANCC and ventricular NCC often is made through serologic testing and imaging features. However, imaging is often nonspecific and/or abnormalities are missed by standard magnetic resonance imaging (MRI) techniques [5]. Enzyme-linked immunoelectrotransfer blot serologic detection has a high sensitivity in subarachnoid NCC; serology, however, simply reflects parasite exposure and cannot be used for assessing disease activity.
SANCC can regrow, causing a relapse of symptoms if inadequately treated [2, 6], and overtreatment is expensive [7], requires a high daily pill burden, and unnecessarily subjects the patient to further, medication-induced side effects. Treatment endpoints for clinicians to follow over the course of therapy are not readily available. A high-volume center demonstrated that 72% of patients with subarachnoid disease do not have complete resolution of cysts on MRI following a durable cure [2]. Thus, imaging is not reliable in indicating when subarachnoid disease has been cured and treatment can stop. There are 2 existing antigen detection assays, both of which detect circulating excretory secretory antigens of Taenia spp [8, 9], and 1, termed HP10, detects antigen levels that have been shown to correlate with disease activity in subarachnoid disease [10], but it is not widely available. The B158/B60 Antigen test, available commercially for research only, is generally believed to behave similarly to the HP10 antigen test, although the utility of this assay awaits assessment in more than the handful of patients described in cross-sectional studies [11, 12].
Real-time quantitative polymerase chain reaction (qPCR) is an attractive platform for biomarker and diagnostic development, given its reproducibility, specificity, scalability, and the ability to utilize the test on any tissue in a human or nonhuman sample following DNA extraction. Here, we describe the identification of multiple, highly repetitive T. solium sequences interspersed throughout the T. solium genome and the development of highly sensitive and highly specific qPCR methods for their detection. Using the most sensitive qPCR assay and targeting TsolR13 in cerebral spinal fluid (CSF) samples—both in subjects with active subarachnoid and ventricular NCC and in subjects at the time of cure—demonstrated clearly that this can be used as a biomarker to guide therapy.
METHODS
Assay Development
Quantitative Polymerase Chain Reaction Target Determination
Following sequencing of the Taenia solium genome (National Center for Biotechnology Information [NCBI] accession number PRJNA183343), RepeatExplorer was used to identify the most highly abundant, repetitive sequences through the mining of raw sequences based on the approach described by Pilotte et al [13]. There were 13 sequences found with >1000x coverage and a minimum length of 70 base pairs (Supplementary Table 1). These sequences were used to design qPCR oligonucleotides using the PrimerQuest Tool (Integrated DNA Technology, www.idtnda.com), and custom oligonucleotides were synthesized (Integrated DNA Technologies, Inc., Coralville, IA; Supplementary Table 2).
Using the Basic Local Alignment Search Tool (BLAST), no highly similar sequences corresponding to TsolR11, TsolR12, or TsolR13 were identified in any cestode or trematode, including the closely related species Taenia crassiceps, Taenia saginata, and Echinococcus spp. If the stringency of the BLAST analysis was reduced slightly, TsolR12 was shown to have some sequence relatedness to Taenia asiatica.
Oligonucleotide Testing and Quantitative Polymerase Chain Reaction Conditions
The target testing, validation, and reporting was in keeping with the Minimum information for publication of quantitative real-time PCR experiments guidelines [14]. The 13 oligonucleotide assay sets were tested alone and in combination using T. solium genomic DNA. Of the 10 assays, 3—TsolR11, TsolR12, and TsolR13 (Table 1)—were found to be more sensitive than the others. These 3 performed very similarly in sensitivity, with TsolR12 having slightly higher quantification cycle (Cq) values at the low end of detection (Figure 1). Published qPCR assays for pTsol 9 [15] and for the T. solium intergenic transcribed spacer (ITS) [16] were also compared using the same conditions used for TsolR11, TsolR12, and TsolR13 (described below).
Table 1.
Oligonucleotide Sequences
| Name | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) | Probe Sequence FAM-5’—ZEN™—3’IABkFQ | Amplicon Size | Comment |
| TsolR11 | GACTGACTGACTGACTGACTTG | ACAGCCTGACAGCTTGAC | TGACATGTGbCCGCATTGTTGTCAC | 98 | … |
| TsolR12 | CTTGTCACACGCCTCACT | CCGCATTGTTGTCACTTGTC | ACTGACTGAbCTGACTGACTGACTGACT | 75 | Detects Taenia saginataa |
| TsolR13 | AGTCAGTCAGTCAGTCAGTCA | CTGTCAAGCTGTCAGGCTGT | TGTCAGTGAbGGCGTGTGACAAGAC | 71 | … |
Sequences used in the most sensitive assays targeting repetitive elements for quantitative polymerase chain reaction detection of Taenia solium. Number of repeats is based on coverage.
aAlso possibly Taenia asiatica, based on Basic Local Alignment Search Tool results, not proven experimentally.
bZEN™ insert location.
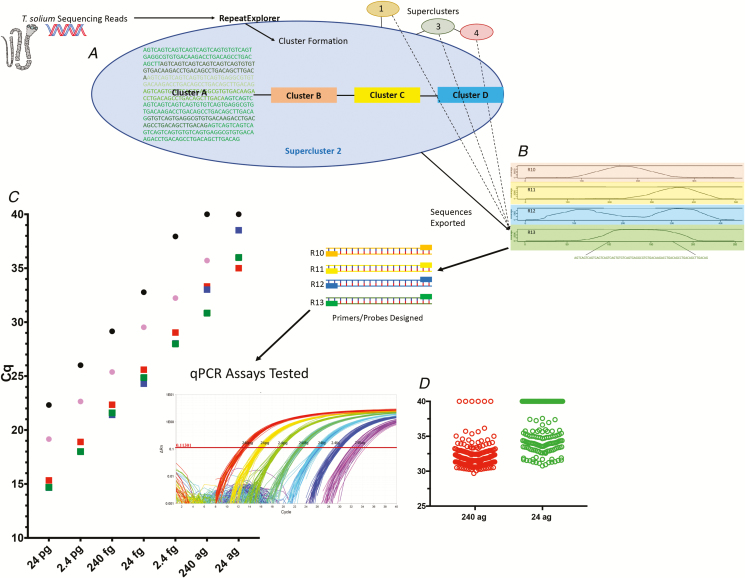
Figure 1.
Workflow of qPCR discovery and testing. A, Raw Taenia solium sequencing reads are clustered by similarity with RepeatExplorer, and result in outputs with variable repeat coverage. B, There are 4 representative, clustered contigs (contiguous segments of overlapping DNA) with greater than 1000x coverage and >70 base pairs shown, with the coverage across the genome on the y axes and the contours of the various nucleotides across the contigs on the x axes. Below the bottom contig (Tsol R13) is the sequence selected for qPCR assay development. C, Comparison of detection of genomic DNA at various concentrations (x axis), using qPCR, for TsolR11 (red squares), TsolR12 (blue squares [hidden underneath other squares in some cases]), TsolR13 (green squares), the ITS [16] (black circles), and pTsol9 [15] (pink circles), plotted as a function of Cq. The insert shows amplification plots of T. solium genomic DNA from 240 pg to 24 ag using Tsol13R run in replicates of 48 each. D, Limit of detection of TsolR13-based qPCR using 188 identical wells at inputs of 240 ag (red circles) and 24 ag (green circles). Abbreviations: ag, attograms; ITS, intergenic transcribed spacer; qPCR, quantitative polymerase chain reaction.
Genomic DNA samples from humans, Taenia crassiceps, Taenia saginata, and Schistosoma mansoni were also used as templates for specificity in TsolR11-, TsolR12-, and TsolR13-based qPCR assays. TsolR11 and TsolR13 could not be amplified from any of these targets; Tsol12R, however, did amplify T. saginata genomic DNA. The efficiency of each assay was extrapolated from a standard curve of 10-fold dilutions of genomic T. solium DNA (24 picograms–2.4 femtograms), performed in replicates of 6. Given the specificity and high degree of efficiency, Tsol13R was identified as the most optimal target for qPCR assays.
The limits of detection for TsolR13 were determined by performing 188 replicates at 240 ag and 24 ag. A limit of detection was defined as a >95% detection rate at a given genomic DNA concentration.
Assay Validation Using Patient Samples
Samples
All samples were obtained as part of National Institute of Allergy and Infectious Diseases’ Institutional Review Board–approved protocols (85-I-0127 for NCC, 97-I-96 (Evaluation, treatment, and monitoring of patients with known or suspected parasitic infections) or 09-I-N178 (Research use of stored human specimens)). As part of the subjects’ routine clinical care, serum, plasma, and (for subarachnoid and ventricular NCC) CSF samples were collected and stored at −80°C. Informed written consent was obtained from all subjects.
Sample Extraction
DNA from CSF was initially extracted using a volume 200 μL using a QIAamp DNA Mini kit (Qiagen catalog number 51306). Results using this kit were found to be equivalent to the use of 50 μL of CSF using the QIAamp MinElute ccfDNA kit (Qiagen, catalog number 55204). DNA from CSF was then extracted with either of these methods.
Plasma was extracted with the QIAamp MinElute ccfDNA kit, from 2 mL volumes. Serum was also tested for some samples for the purposes of comparison and was found to result in quantification cycle values 0–3 more than using plasma (Supplementary Figure 1). For some of the patients not infected with T. solium helminth and for whom plasma was not available, 2mL of serum was substituted. All DNA was eluted in 50 μL of ultrapure water (Invitrogen, catalog number 10977015)
All starting material was spiked with 0.4 nanogram of an internal amplification control plasmid [17] and was amplified in parallel with other targets to exclude false negatives due to qPCR inhibition.
Patient Sample Quantitative Polymerase Chain Reaction
All samples, as well as positive and negative controls, were run in a total of 10 µL with Taqman Fast Advanced Master Mix 2x Buffer (Thermo Fisher, 4444558): each primer had a final concentration of 900 nM, probe of 250 nM, and 2 μL of extracted DNA. Reactions were carried out in 384 well plates (Applied Biosystems, catalog number 4343370) with adhesive seals (Applied Biosystems, catalog number 4313663) on the Viia 7 Real-Time PCR System (Thermo Fisher, 4453535) with an initial 95°C 20-second incubation, followed by 2-step PCR cycling between 95°C for 1 second and 60°C for 20 seconds, for 40 cycles. Each sample was run in triplicate.
Diagnosis
All patients with NCC in this cohort presented with neurologic symptoms and were diagnosed with subarachnoid and/or ventricular disease based on a positive serum enzyme-linked immunoelectrotransfer blot test for T. solium (performed at the Centers for Disease Control, Atlanta, GA) and the demonstration of cysts or membranes in the subarachnoid spaces or ventricles on MRI, best seen on balanced fast field echo sequences.
Definitions
Since the objective was to develop a biomarker that would reflect any live T. solium parasite that would require the use of anthelmintic drugs, the “active disease” time point was defined to capture the time where the organism was likely to be living/proliferating. The active disease sample was obtained at any time before or during the first 30 days of anthelmintic treatment (praziquantel and/or albendazole) or, for surgically resected ventricular disease, any time prior to surgical resection. Previously treated subarachnoid disease was considered to have recurred when a new growth of a previous lesion or a new lesion was visualized by MRI or when new symptoms occurred that were accompanied by CSF pleocytosis that normalized with intensive anthelmintic/adjunctive treatment. Subjects were considered to have been cured at 2 weeks prior to stopping anthelmintic therapy or later. Since there is no current gold standard for indicating a cure, patients were retrospectively classified as cured from subarachnoid disease (or ventricular disease that was medically managed) following at least 3 years of posttreatment observation, with an annual MRI demonstrating no lesion growth, no areas of new involvement, and no recurrence of symptoms associated with an increase in CSF white blood cell. This time period was chosen based on our experience with the longest time to recurrence while under medical care. Those with disease incidences limited to the ventricles were considered cured immediately following complete surgical removal of the cyst [3].
Taenia solium Antigen Testing
CSF and serum samples subjected to qPCR testing were also tested for circulating T. solium antigens (TsAgs) by a commercially available enzyme-linked immunosorbent assay (ApDia, Turnhout, Belgium) that detects B158/B60 [8], following the manufacturer’s instructions.
Statistical Analyses
All statistics were performed by Prism GraphPad V7. Sensitivity and specificity calculations were performed using receiver operator characteristic curves. Unpaired samples were compared using the Mann-Whitney test and paired analyses were performed the using Wilcoxon matched-pairs signed rank test.
RESULTS
Assay Development
Of 13 candidate repeats in the T. solium genome, 2 (named TsolR11 and TsolR13) were superior in sensitivity and specificity (Figure 1). A third (R12) was comparable to the others but also amplified T. saginata genomic DNA. Both TsolR11 and TsolR13 failed to amplify genomic DNA from the closely related species of T. crassiceps and T. saginata, as well as genomic DNA from Schistosoma mansoni, giving the assay an analytical specificity of 100%. Because of a slightly better efficiency in qPCR (98.25%, see Supplementary Table 3), TsolR13 was chosen to move forward with clinical testing. Compared with 2 previously published qPCR targets (ITS and pTsol9), TsolR13 detected T. solium genomic DNA at a 10-fold lower input gDNA concentration than pTsol9 (Figure 1) and a 100-fold lower concentration than the ITS targeted sequence. When run in parallel, amplification occurred at Cq values 7–10 cycles lower than the ITS targeted sequence and 4–5 cycles lower than the pTsol9 target. The limit of detection (analytical sensitivity) for TsolR13 was 240 ag (97.3% amplification rate), although concentrations as low as 24 ag were frequently detected (57.98% amplification rate).
TsolR13 Quantitative Polymerase Chain Reaction Performance in Clinical Samples
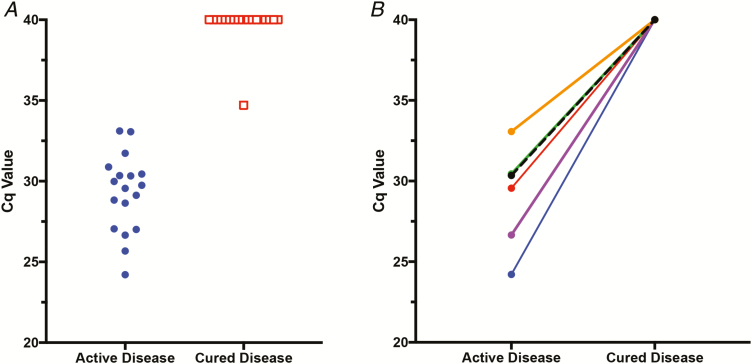
At time points that met the criteria for active and/or cured disease, 43 patients with subarachnoid and/or ventricular NCC had plasma and/or CSF samples taken. Males comprised 63.6% of the total subject pool. Subarachnoid involvement of the sylvian fissure and/or basilar area of the brain was involved in 68.2% of patients, and ventricular disease was found in the remainder (31.8%). Most patients (93.2%) were from the Americas, and 3 (6.8%) were from Southeast Asia. A total of 82 samples from these subjects were included in this study. There were 6 subjects who had paired CSF samples taken during both active and cured disease time points and 5 who had paired plasma samples: all the other samples were unpaired (see Figure 2 for a sample breakdown and Supplementary Table 5 for sample details). Of the 18 CSF samples from those with an active disease, 18 were positive for T. solium DNA by qPCR using TsolR13. Of the 18 CSF samples taken from those with a cured disease, 17 were negative by qPCR using TsolR13 (Figure 3A). Thus, in CSF, qPCR had a diagnostic sensitivity of 100% (81.5–100%) in identifying those with active NCC disease. In this patient population, having a negative TsolR13 qPCR had a predictive value of 94.4% (confidence interval 72.7–99.9%) in predicting a cure. Of the CSF samples tested, there were 6 paired active/cured samples (Figure 3B), and all had detectable T. solium DNA (Cq value <40) during the active disease that became undetectable (no amplification by Cq 40) at the time of cure (P = .0312).
Figure 2.
Sample breakdown. From 43 patients, there were 36 CSF and 46 plasma samples. There were 6 patients that had paired CSF samples and 5 patients that had paired plasma samples from both active and cured time points; the remainder were unpaired. The total number of samples for each time point is given in a breakdown of whether the samples came from patients with subarachnoid or ventricular disease. The median time from sample collection to therapy is given for each group, with a range. A negative number indicates the number of days prior to starting (for active disease) or stopping (for cure) anthelmintics that the sample was collected; a positive number indicates the number of days after starting (active) or stopping (cure) anthelmintics that the sample was collected. Abbreviation: CSF, cerebral spinal fluid.
Figure 3.
Performance of qPCR on CSF. A, Results of performing a TsolR13 qPCR assay on unpaired CSF samples from patients during an infection with active NCC (n = 18, blue circles) and during cured NCC (n = 18, red squares), plotted as a function of Cq (y axis). A Cq of 40 is considered negative; less than 40 is considered positive. B, Results of TsolR13 qPCR on 6 subjects with paired CSF samples (subset of panel A samples) during active and cured (x axis) time periods, plotted as a function of Cq. Abbreviations: CSF, cerebral spinal fluid; NCC, neurocysticercosis; qPCR, quantitative polymerase chain reaction.
In testing plasma samples from patients with subarachnoid/ventricular NCC, as shown in Figure 4A, 12 of 16 samples from those with an active disease were positive by qPCR. Plasma or serum samples from 25 subjects with other non-NCC parasitic infections (eg active echinococcosis [n = 5], schistosomiasis [n = 2], and noncestode parasites [n = 18]) were tested by TsolR13. All (100%) were negative using TsolR13 qPCR. Thus, in plasma qPCR, TsolR13 had a diagnostic sensitivity for active CNS infection of 76.5% (50.1–93.2%) and a specificity in establishing the diagnosis of 100% (86.3–100%). As a biomarker of a cure, 26 of 30 plasma samples in the cured patients were negative by qPCR, giving a predictive value in predicting a cure in this population of 86.7% (70.3–94.7%).
Figure 4.
Performance of qPCR on plasma. A, Results in Cq value (y axis) of unpaired plasma samples from active NCC (n = 16, blue dots), cured NCC (n = 30, red squares), and non-NCC (echinococcosis, n = 5; schistosomiasis, n = 2; non–cestode-infected n = 18, green circles) subjects tested by TsolR13 qPCR. A Cq of 40 is considered negative; less than 40 is considered positive. B, There were 5 subjects who had paired plasma samples (subset of panel A) from active and cured time points (x axis), with the results of TsolR13 qPCR shown as a function of the Cq value. Abbreviations: NCC, neurocysticercosis; qPCR, quantitative polymerase chain reaction.
Active Neurocysticercosis Versus Cure: Comparison with Antigen Testing
All NCC patient samples available (4 CSF samples were not available for TsAg testing) had TsAg assays performed, and the results were compared to TsolR13 qPCR (Figure 5). In the setting of active NCC, 17 of 17 (100%) CSF samples were correctly identified as positive by both qPCR and TsAg. In distinguishing active from cured infections in the CSF compartment, 14 of 15 (93.3%) samples were correctly identified as being negative by qPCR and 12 of 15 (80%) were correctly identified by TsAg. In the plasma (qPCR) or serum (TsAg) samples of those with active disease, 13 of 16 (81.3%) were correctly identified as active with a positive qPCR test and 12 of 16 (75%) were correctly identified by TsAg. In those cured, qPCR results were negative in 26 of 30 (86.7%) patients, and TsAg results were negative in 24 of 30 (80%). Therefore, in each comparison, qPCR performed equivalent to or slightly better than TsAg in predicting active and cured NCC.
Figure 5.
Comparison of qPCR to antigen detection. Performance of TsolR13 qPCR, compared to TsAg B158/B60 enzyme-linked immunosorbent assay detection (ApDia), in CSF and plasma/serum samples at the time of active and cured subarachnoid or ventricular neurocysticercosis. Red indicates test positivity; blue indicates test negativity. A, In the CSF, all 17 (100%) of those with active disease were positive by qPCR and TsAg. B, In the CSF of those cured, 14 of 15 (93.3%) samples were negative by qPCR and 12 of 15 (80%) were negative by TsAg. C, In the plasma (qPCR) or serum (TsAg) of those with active disease, 13 of 16 (81.3%) were positive by qPCR and 12 of 16 (75%) by TsAg. D, In those cured, qPCR was negative in 26 of 30 (86.7%) patients and TsAg was negative in 24 of 30 (80%) patients. Note that 1 active CSF and 3 cured CSF samples were not available for TsAg testing, so were excluded from this comparison. Abbreviations: CSF, cerebral spinal fluid; qPCR, quantitative polymerase chain reaction; TsAg, Taenia solium antigen.
Durability of Response Following Cure
There were 3 patients who had CSF available from multiple time points following the active sample, and they were assessed to observe the durability of the response (Supplementary Table 4). Of these, 1 patient (patient #3) remained positive at the time of cure (Figure 3A) and was first found to be negative by qPCR at Day 741 following treatment. Notably, the antigen assay remained positive at Day 741 and did not become negative until the following lumbar puncture (Day 2134). Once the CSF turned negative for all 3 patients, all further CSF taken remained negative by qPCR, as well as by TsAg. Of the 25 patients with plasma taken longitudinally following the initial sample, only 15 (60%) were negative by qPCR at all subsequent time points. Interestingly, 7 of 25 (28%) patients had plasma qPCR testing that became positive some time (from 187 to 797 days) after having had a negative qPCR.
DISCUSSION
Here, we describe the development and validation of TsolR13, a highly sensitive and specific qPCR assay based on a novel repeat for T. solium. It demonstrated more sensitivity than previous qPCR assays and showed 100% specificity when tested against closely related species (including T. saginata and T. crassiceps), as well as in clinical samples from patients with Echinococcus or Schistosoma infections.
Based on our data, the use of TsolR13-directed qPCR is most sensitive (100%) in detecting active subarachnoid/ventricular NCC in the CSF compartment. However, overall sensitivity in plasma samples in making the diagnosis of active subarachnoid or ventricular NCC was 76.5%. Despite genetic differences found in T. solium from the Americas and Asia [18], TsolR13 was able to detect cell-free DNA from subjects with diseases acquired in both locations. As a test of cure, 94.8% of patients shown to have a durable cure from ventricular or SANCC were negative by qPCR TsolR13 in the CSF, compared with 80% correctly testing as negative by TsAg in the CSF. Thus, the use of qPCR appears to be a very promising tool to use as a CSF-based test of cure, with potential improved specificity over TsAg detection.
Plasma/serum TsolR13 qPCR negativity as a test of cure showed a reasonable (80.7%) predictive value. However, because during the long-term follow-up 28% of patients had detectable T. solium DNA in their plasma samples following a cure, qPCR assessments of plasma may not be useful for long-term monitoring of disease activity. However, with a 81.3% sensitivity and 100% specificity in diagnosing NCC in this population, testing the plasma can be useful for establishing the diagnosis of NCC. Given that, in 1 patient, long-term CSF collection following a cure continued to show the absence of T. solium cell-free DNA (cfDNA) despite the presence of cfDNA in the plasma, these 2 compartments appear to shed cfDNA independently. The presence of peripheral blood cfDNA may reflect an eventual breakdown of calcified T. solium cysts in the muscle, as DNA is known to be well preserved in calcifications [19–22].
Nearly all patients with subarachnoid/ventricular NCC attracted clinical attention due to the development of symptoms [23], and that was the patient population we examined for this study. However, interestingly, 1 patient who was found incidentally to have subarachnoid disease after a brain MRI for an unrelated injury had CSF found to be negative by qPCR for TsolR13, as was the patient’s plasma. After starting anthelmintic treatment, the plasma qPCR became positive (CSF was not collected again). This likely indicates that T. solium cell-free DNA does not circulate in an undisturbed cyst and requires damage (or an inflammatory response) for DNA leakage.
Previous studies in NCC patients have not been able to detect DNA in the blood by qPCR, and sensitivities in the CSF have ranged from 83–92% in those with subarachnoid/ventricular disease [15] and from 16–44% with intra- and extraparenchymal disease [24]. The superior sensitivity shown here is likely due to both improvements in cell-free DNA extraction techniques and the improved sensitivity of targeting the highly repetitive sequence of TsolR13.
Further areas that deserve study with the TsolR13 qPCR assay are its performance in patients with parenchymal disease, stool testing for tapeworm detection (distinguishing T. solium from T. saginata), and epidemiologic studies in surveying pig infections. Nevertheless, we have developed a widely adaptable, scalable, sensitive, and specific qPCR assay that may be used for both diagnosing and monitoring subarachnoid and ventricular NCC.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Division of Intramural Research, National Institutes of Health.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 2. Osorio R, Carrillo-Mezo R, Romo ML, et al. Factors associated with cysticidal treatment response in extraparenchymal neurocysticercosis. J Clin Pharmacol 2019; 59:548–56. [DOI] [PubMed] [Google Scholar]
- 3. Nash TE, Ware JM, Mahanty S. Intraventricular neurocysticercosis: experience and long-term outcome from a tertiary referral center in the United States. Am J Trop Med Hyg 2018; 98:1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 2018; 98: 945–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrillo Mezo R, Lara García J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop 2015; 152:60–5. [DOI] [PubMed] [Google Scholar]
- 6. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther 2011; 9:123–33. [DOI] [PubMed] [Google Scholar]
- 7. Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs–implications for patients and policymakers. N Engl J Med 2014; 371:1859–62. [DOI] [PubMed] [Google Scholar]
- 8. Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 1992; 22:471–7. [DOI] [PubMed] [Google Scholar]
- 9. Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol 1989; 11:351–70. [DOI] [PubMed] [Google Scholar]
- 10. Fleury A, Garcia E, Hernández M, et al. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLOS Negl Trop Dis 2013; 7:e2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia HH, Dorny P, Castillo Y, et al. Circulating antigen levels follow post-treatment evolution of subarachnoid neurocysticercosis. J Neuroparasitol 2010; 1:1–3. [Google Scholar]
- 12. Rodriguez S, Dorny P, Tsang VC, et al. ; Cysticercosis Working Group in Peru Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 2009; 199:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilotte N, Papaiakovou M, Grant JR, et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLOS Negl Trop Dis 2016; 10:e0004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55:611–22. [DOI] [PubMed] [Google Scholar]
- 15. Yera H, Dupont D, Houze S, et al. Confirmation and follow-up of neurocysticercosis by real-time PCR in cerebrospinal fluid samples of patients living in France. J Clin Microbiol 2011; 49:4338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Praet N, Verweij JJ, Mwape KE, et al. Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Trop Med Int Health 2013; 18:608–14. [DOI] [PubMed] [Google Scholar]
- 17. Deer DM, Lampel KA, González-Escalona N. A versatile internal control for use as DNA in real-time PCR and as RNA in real-time reverse transcription PCR assays. Lett Appl Microbiol 2010; 50:366–72. [DOI] [PubMed] [Google Scholar]
- 18. Ito A, Yanagida T, Nakao M. Recent advances and perspectives in molecular epidemiology of Taenia solium cysticercosis. Infect Genet Evol 2016; 40:357–67. [DOI] [PubMed] [Google Scholar]
- 19. Marmion BP, Sukocheva O, Storm PA, et al. Q fever: persistence of antigenic non-viable cell residues of Coxiella burnetii in the host–implications for post Q fever infection fatigue syndrome and other chronic sequelae. QJM 2009; 102:673–84. [DOI] [PubMed] [Google Scholar]
- 20. Branger S, Casalta JP, Habib G, Collard F, Raoult D. Streptococcus pneumoniae endocarditis: persistence of DNA on heart valve material 7 years after infectious episode. J Clin Microbiol 2003; 41:4435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agerholm JS, Jensen TK, Agger JF, Engelsma MY, Roest HI. Presence of Coxiella burnetii DNA in inflamed bovine cardiac valves. BMC Vet Res 2017; 13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohishi K, Takishita K, Kawato M, et al. Molecular evidence of new variant brucella in North Pacific common minke whales. Microbes Infect 2004; 6:1199–204. [DOI] [PubMed] [Google Scholar]
- 23. Bazan R, Hamamoto Filho PT, Luvizutto GJ, et al. Clinical symptoms, imaging features and cyst distribution in the cerebrospinal fluid compartments in patients with extraparenchymal neurocysticercosis. PLOS Negl Trop Dis 2016; 10:e0005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rottbeck R, Nshimiyimana JF, Tugirimana P, et al. High prevalence of cysticercosis in people with epilepsy in southern Rwanda. PLOS Negl Trop Dis 2013; 7:e2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.