Figure 2.
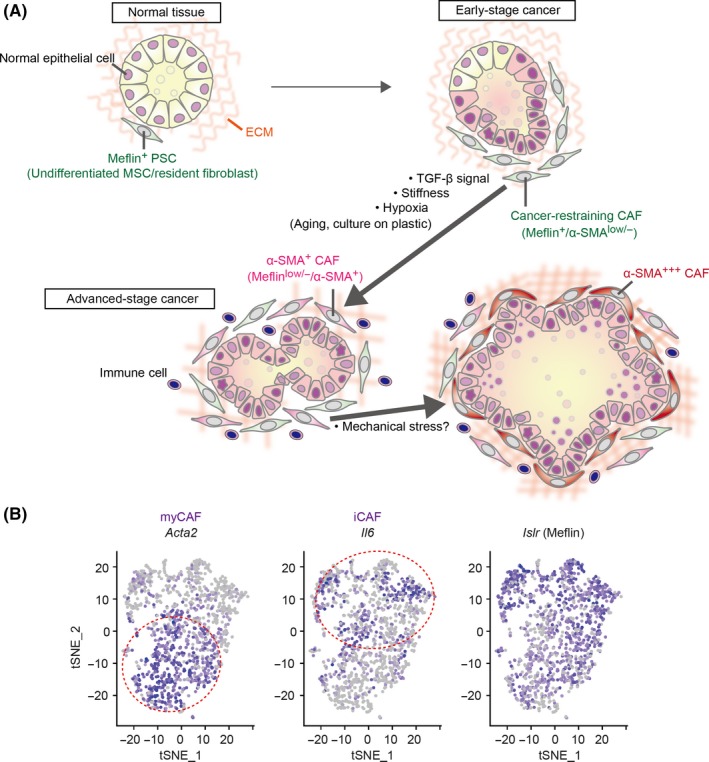
A possible mechanism underlying cancer‐associated fibroblast (CAF) heterogeneity. A, In the pancreas, Meflin marks pancreatic stellate cells (PSC), undifferentiated mesenchymal stem/stromal cells (MSC) and resident fibroblasts that localize around the acini, ducts and the islets of Langerhans. In the early stages of cancer development, including ADM and preinvasive (PanIN) stages, Meflin+ CAF or PSC that express low levels of α‐SMA start to proliferate around neoplastic cells, which we termed cancer‐restraining cancer‐associated fibroblasts (rCAF). Our recent studies showed that Meflin expression is downregulated by TGF‐β, stiff substrate and hypoxia, which we speculate are major factors that drive the differentiation of rCAF into α‐SMA+ and Meflin−/low CAF in advanced‐stage cancer. Aging and ex vivo culture of cells also cause downregulation of Meflin expression, although the relevance of these factors in cancer development and progression has not been demonstrated. In pancreatic ductal adenocarcinoma (PDAC), some CAF that are predominantly positive for α‐SMA preferentially localize immediately adjacent to the tumor glands, although there is currently no evidence that these CAF are derived from Meflin+ cells. The effect of mechanical stress imposed by the expanded tumor glands on α‐SMA expression in the CAF should also be considered. B, t‐distributed stochastic neighbor embedding (t‐SNE) plot showing the myCAF, iCAF and Meflin+ CAF subpopulations identified by scRNA‐seq of all cells isolated from tumors of a PDAC mouse model. Each dot is a CAF, and the intensity of the purple represents the expression level of the indicated genes. Reprinted from ref. 50, Copyright (2019), with permission from the American Association for Cancer Research
